
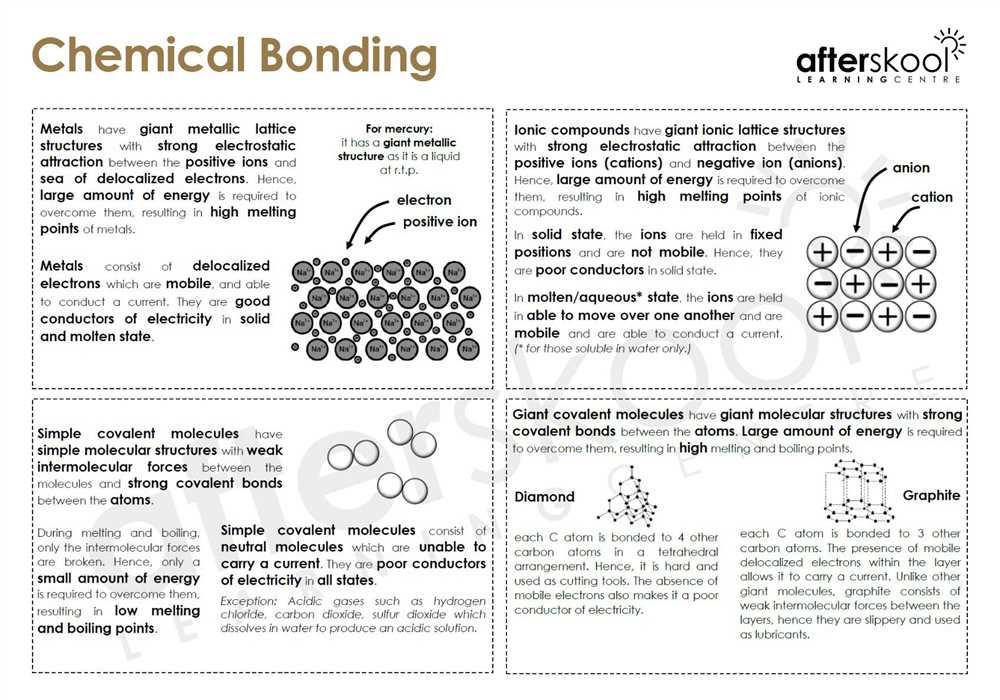
In the world of chemistry, understanding the basics of chemical bonding is crucial. One important type of chemical bond is a covalent bond. Covalent bonds form when two atoms share electrons in order to achieve a full outer electron shell. This type of bonding allows atoms to become more stable and form molecules.
When it comes to covalent bonds, it is important to understand how to determine the number of bonds that can be formed between two atoms. This can be determined by looking at the number of valence electrons each atom has. Valence electrons are the electrons in the outermost energy level of an atom. The octet rule states that atoms tend to form bonds in such a way that each atom ends up with a full outer electron shell, which is usually eight valence electrons.
In the worksheet “Bonding Basics Covalent Bonds,” students are given various chemical formulas and are asked to determine the number of covalent bonds that can be formed between the atoms. By understanding the number of valence electrons each atom has and applying the octet rule, students can successfully determine the number of bonds. This worksheet serves as a useful tool for reinforcing the concept of covalent bonding and allows students to practice their skills in counting valence electrons and determining the number of bonds that can be formed.
Bonding Basics – Covalent Bonds Worksheet Answers
Covalent bonds occur when two or more atoms share electrons in order to achieve a stable electron configuration. This type of bonding typically occurs between nonmetal atoms. In covalent bonding, the shared electrons are represented by a dash (–) or by a pair of dots (:) between the atoms. The Lewis structure or electron dot diagram can be used to represent covalent bonds.
Here are the answers to the Covalent Bonds Worksheet:
- 1. The chemical formula for water is H2O. This means that each water molecule contains two hydrogen atoms and one oxygen atom.
- 2. The chemical formula for methane is CH4. This means that each methane molecule contains one carbon atom and four hydrogen atoms.
- 3. The chemical formula for ammonia is NH3. This means that each ammonia molecule contains one nitrogen atom and three hydrogen atoms.
- 4. Carbon dioxide has the chemical formula CO2. This means that each carbon dioxide molecule contains one carbon atom and two oxygen atoms.
- 5. The chemical formula for sulfuric acid is H2SO4. This means that each sulfuric acid molecule contains two hydrogen atoms, one sulfur atom, and four oxygen atoms.
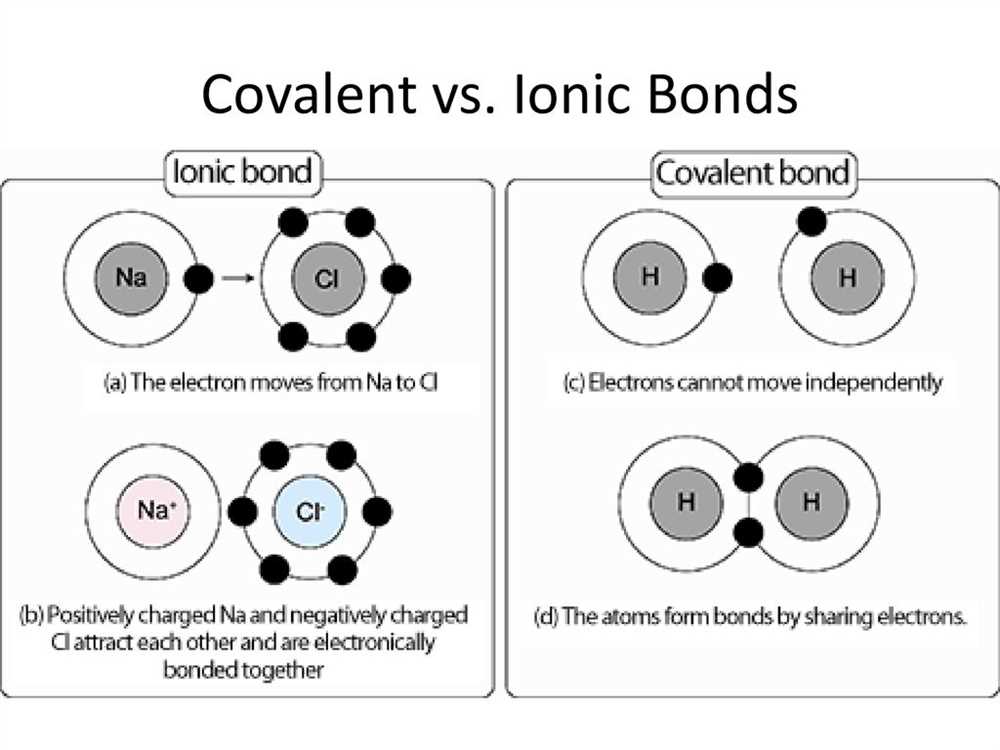
Covalent bonds form between atoms with high electronegativity differences and can be either polar or nonpolar. If the electronegativity difference is small, the bond is considered nonpolar, while if the difference is large, the bond is considered polar. In polar covalent bonds, the electrons are unequally shared between the atoms, resulting in a partial positive charge on one atom and a partial negative charge on the other.
Overall, covalent bonds are important for the formation of molecules and compounds, as they allow atoms to share electrons and achieve stability. Understanding the chemical formulas and structures of covalent compounds can help in predicting their properties and behavior in various chemical reactions.
What are Covalent Bonds?
In chemistry, a covalent bond is a strong chemical bond that is formed when two atoms share a pair of electrons. It is one of the most common types of chemical bonds in nature. Covalent bonds are usually formed between nonmetallic elements and involve the sharing of electrons in order to achieve a more stable electron configuration.
Covalent bonds are formed through the overlap of atomic orbitals. Each atom contributes one or more electrons to the shared electron pair, allowing both atoms to achieve a full outer electron shell. The shared electrons are attracted to both nuclei, creating a strong bond between the atoms.
In a covalent bond, the electrons are not transferred from one atom to another as in ionic bonds, but rather they are shared between the atoms. This sharing of electrons allows atoms to form stable molecules and compounds. The strength of a covalent bond depends on the number of shared electrons and the distance between the nuclei of the atoms involved.
Covalent bonds can be either polar or nonpolar, depending on the electronegativity difference between the atoms involved. In a polar covalent bond, the electrons are not shared equally and there is a partial separation of charges, resulting in a slightly positive and a slightly negative end. In a nonpolar covalent bond, the electrons are shared equally between the atoms.
Covalent bonds play a crucial role in the formation of organic compounds, such as carbohydrates, lipids, proteins, and nucleic acids. These compounds are the building blocks of life and are essential for the functioning of living organisms. Understanding the nature of covalent bonds is therefore fundamental in the study of chemistry and biology.
How do Covalent Bonds Form?
Covalent bonds are formed when atoms share electrons in order to achieve a stable electron configuration. In a covalent bond, two atoms come together and each contributes one or more electrons to a shared electron pair. This shared electron pair forms a bond between the two atoms, creating a molecule.
Covalent bonds can form between atoms of nonmetals or between atoms of nonmetals and hydrogen. These types of bonds are usually formed between atoms that have a similar electronegativity, or tendency to attract electrons. This allows for a more even distribution of electrons between the two atoms involved in the bond.
In a covalent bond, the shared electrons are attracted to both nuclei of the bonded atoms. This attraction keeps the atoms together and forms a stable molecule. The strength of a covalent bond depends on the number of shared electrons and the distance between the nuclei of the bonded atoms.
Covalent bonds play a crucial role in the formation of many substances, including water, carbon dioxide, and DNA. They are important for the stability and functionality of these molecules, allowing them to interact with other substances and carry out their biological or chemical functions.
Understanding Bond Length and Bond Strength
When it comes to understanding covalent bonds, two important factors to consider are bond length and bond strength. Bond length refers to the distance between the nuclei of two bonded atoms, while bond strength measures the amount of energy required to break the bond.
Bond length: The length of a covalent bond is influenced by several factors, including the size of the atoms involved and the number of shared electrons. Generally, larger atoms have longer bond lengths, as the distance between their nuclei is greater. Additionally, a single covalent bond is longer than a double or triple bond between the same two atoms, as more shared electrons lead to a stronger attraction and shorter bond length.
Bond strength: The strength of a covalent bond is determined by the amount of energy required to break the bond and separate the atoms. In general, shorter bonds are stronger than longer bonds, as the nuclei of the atoms involved are closer together and the shared electrons are held more tightly. Additionally, multiple bonds are stronger than single bonds between the same atoms, as the greater number of shared electrons leads to a stronger attraction.
Understanding bond length and bond strength is crucial in predicting the properties and behavior of covalent compounds. By analyzing these factors, scientists can gain insight into the stability and reactivity of substances, as well as their physical and chemical properties.
Lewis Structures and the Octet Rule
The concept of Lewis structures is a fundamental tool in understanding the bonding behavior of atoms and molecules. Lewis structures are diagrams that represent the arrangement of electrons in a molecule and help determine the type of chemical bonds formed between atoms. One key principle in constructing Lewis structures is the octet rule, which states that most atoms tend to gain, lose, or share electrons in order to achieve a stable electron configuration with eight electrons in their outermost energy level, known as the valence shell.
According to the octet rule, atoms will form covalent bonds by sharing electrons in a way that allows each atom to achieve a full octet. In a covalent bond, the shared pair of electrons forms a bond between the two atoms, resulting in a more stable arrangement of electrons. To represent covalent bonds in a Lewis structure, a line is drawn between the atoms to indicate the pair of shared electrons.
The octet rule also applies to most main group elements, such as carbon, nitrogen, oxygen, and fluorine. However, there are exceptions to the octet rule, particularly for elements in the second period of the periodic table. These elements, known as the group 13 elements (boron, aluminum, etc.), often form covalent compounds with incomplete octets, as they have fewer than eight valence electrons. Additionally, elements beyond the second period, such as sulfur and phosphorus, can accommodate more than eight electrons in their valence shells, violating the octet rule.
In summary, Lewis structures and the octet rule provide a visual representation of how atoms form covalent bonds to achieve a stable electron configuration. Understanding these concepts is essential for predicting the chemical behavior and properties of molecules, as well as for explaining the formation of different types of bonds. While the octet rule generally holds true for many elements, the existence of exceptions reminds us of the complexity of chemical bonding and the need for further exploration and understanding.
Molecular Geometry and Polarity
Molecular geometry refers to the arrangement of atoms in a molecule. It is important to understand molecular geometry as it affects the physical and chemical properties of a compound. The shape of a molecule is determined by the number of electron groups around the central atom. This is known as the electron group arrangement.
One way to predict the molecular geometry is by using the VSEPR (Valence Shell Electron Pair Repulsion) theory. According to this theory, electron groups around the central atom will arrange themselves in a way that minimizes repulsion between them. This results in various molecular shapes such as linear, trigonal planar, tetrahedral, and so on.
Another important concept related to molecular geometry is polarity. Polarity refers to the unequal distribution of electron density in a molecule, resulting in a partial positive charge on one end and a partial negative charge on the other. A molecule can be classified as either polar or nonpolar. Polar molecules have a dipole moment due to the presence of polar bonds or an asymmetrical molecular shape.
Understanding molecular geometry and polarity is crucial in various areas of chemistry, including organic chemistry, chemical bonding, and biochemistry. It helps in predicting the behavior and properties of compounds, as well as understanding their interactions with other molecules. For example, the polarity of a molecule can influence its solubility in different solvents and its ability to form hydrogen bonds.
Key Points:

- Molecular geometry refers to the arrangement of atoms in a molecule.
- VSEPR theory helps in predicting the molecular shape based on the electron group arrangement.
- Polarity refers to the unequal distribution of electron density in a molecule.
- Molecular geometry and polarity are important in understanding compound properties and interactions.
Intermolecular Forces in Covalent Compounds
The study of intermolecular forces is crucial in understanding the behavior of covalent compounds. Intermolecular forces are the attractive forces that exist between molecules, and they play a significant role in determining the physical properties of covalent compounds, such as boiling point and solubility. These forces are weaker than the intramolecular forces that hold atoms together within a molecule, which are covalent bonds in the case of covalent compounds.
There are three main types of intermolecular forces: London dispersion forces, dipole-dipole forces, and hydrogen bonding. London dispersion forces, also known as van der Waals forces, are the weakest and result from temporary fluctuations in electron distribution within a molecule. These forces exist between all molecules, regardless of their polarity. Dipole-dipole forces arise from the attraction between the positive end of one polar molecule and the negative end of another polar molecule. These forces are stronger than London dispersion forces and are present only in polar molecules. Hydrogen bonding, the strongest type of intermolecular force, occurs when a hydrogen atom is bonded to a highly electronegative atom (such as oxygen, nitrogen, or fluorine) and is attracted to another electronegative atom.
Understanding these intermolecular forces is essential in predicting the physical properties of covalent compounds. For example, covalent compounds with stronger intermolecular forces, such as hydrogen bonding, tend to have higher boiling points and are more likely to be soluble in polar solvents. On the other hand, covalent compounds with weaker intermolecular forces, such as London dispersion forces, have lower boiling points and are less likely to dissolve in polar solvents. The presence and strength of these forces also affect the phase behavior of covalent compounds, such as their ability to exist as gases, liquids, or solids at a given temperature and pressure.
Types of Intermolecular Forces:
- London dispersion forces: result from temporary fluctuations in electron distribution within a molecule; exist between all molecules
- Dipole-dipole forces: arise from the attraction between the positive end of one polar molecule and the negative end of another polar molecule; present in polar molecules
- Hydrogen bonding: occurs when a hydrogen atom is bonded to a highly electronegative atom and is attracted to another electronegative atom; strongest type of intermolecular force
Overall, the study of intermolecular forces in covalent compounds helps us understand their behavior and properties. It allows scientists to predict how covalent compounds will interact with other substances and provides insights into their physical characteristics. By understanding these forces, researchers can develop new materials, improve drug delivery systems, and enhance our understanding of the molecular world.