
Stoichiometry is a fundamental concept in chemistry that deals with the quantitative relationships between reactants and products in a chemical reaction. It allows chemists to determine the amount of each substance involved in a reaction and predict the outcome of the reaction.
This article presents the answer key for Chapter 12 of a stoichiometry textbook. The chapter covers topics such as balancing chemical equations, calculating stoichiometric ratios, and performing stoichiometric calculations.
The answer key provided here will help students check their understanding and practice their problem-solving skills. It includes step-by-step solutions to the problems and explanations of the reasoning behind each step. By using this answer key, students can verify if their answers are correct and learn from any mistakes they may have made.
Chapter 12 Stoichiometry Answer Key
In Chapter 12 of the chemistry textbook, stoichiometry is explored in depth. Stoichiometry is the calculation of the quantities of reactants and products in chemical reactions based on balanced chemical equations. It provides a way for chemists to understand the relationship between the amount of substance used and the amount of product formed in a chemical reaction.
The answer key for Chapter 12 provides students with step-by-step solutions to the practice problems and exercises in the textbook. It serves as a valuable tool for students to check their work and ensure they are approaching the calculations correctly. The answer key includes calculations for determining the molar ratio, converting between moles and grams, and solving stoichiometric problems using the mole-mole ratio.
One key concept covered in Chapter 12 is the concept of limiting reactants. The answer key helps students understand how to identify the limiting reactant in a chemical reaction and calculate the maximum amount of product that can be formed. It also provides guidance on how to calculate the excess reactant and determine the amount of the excess reactant that remains unreacted.
The answer key also explains the concept of percent yield, which is the ratio of the actual yield of a reaction to the theoretical yield. It helps students understand how to calculate percent yield and interpret its significance in terms of the efficiency of a chemical reaction.
In conclusion, the Chapter 12 Stoichiometry Answer Key provides students with a comprehensive guide to solving stoichiometric problems. It helps students understand the calculations involved in determining reactant and product quantities, identifying limiting reactants, calculating percent yield, and interpreting the efficiency of a chemical reaction.
What is Stoichiometry?

Stoichiometry is a branch of chemistry that deals with the quantitative relationships between reactants and products in a chemical reaction. It allows us to determine the amount of substances consumed or produced in a reaction by using balanced chemical equations and mole ratios.
In stoichiometry, the concept of the mole is crucial. The mole is a unit used to measure the amount of a substance and is defined as the number of particles, such as atoms or molecules, in a given sample. By knowing the molar ratios of reactants and products in a chemical equation, we can calculate the number of moles of each substance involved in a reaction.
Stoichiometry is essential in various applications, including determining the theoretical yield of a reaction (the maximum amount of product that can be obtained), calculating the percent yield (the actual amount of product obtained compared to the theoretical yield), and determining the limiting reactant (the reactant that is completely consumed in a reaction).
To perform stoichiometric calculations, one must start with a balanced chemical equation that represents the reaction. The coefficients in the equation indicate the mole ratios between the reactants and products. These ratios can be used to convert between different units of measurement, such as grams, moles, and liters.
Example:
Consider the reaction between hydrogen gas (H2) and oxygen gas (O2) to produce water (H2O):
2H2 + O2 -> 2H2O
If we have 4 moles of hydrogen gas, we can use the ratio of 2 moles of hydrogen gas to 2 moles of water to calculate the amount of water produced. In this case, we would expect to produce 4 moles of water.
Stoichiometry is a fundamental concept in chemistry and provides a basis for understanding the quantitative aspects of chemical reactions. It allows chemists to predict and control the outcome of reactions, as well as optimize processes in various industries, such as pharmaceuticals, agriculture, and energy production.
How to Balance Chemical Equations
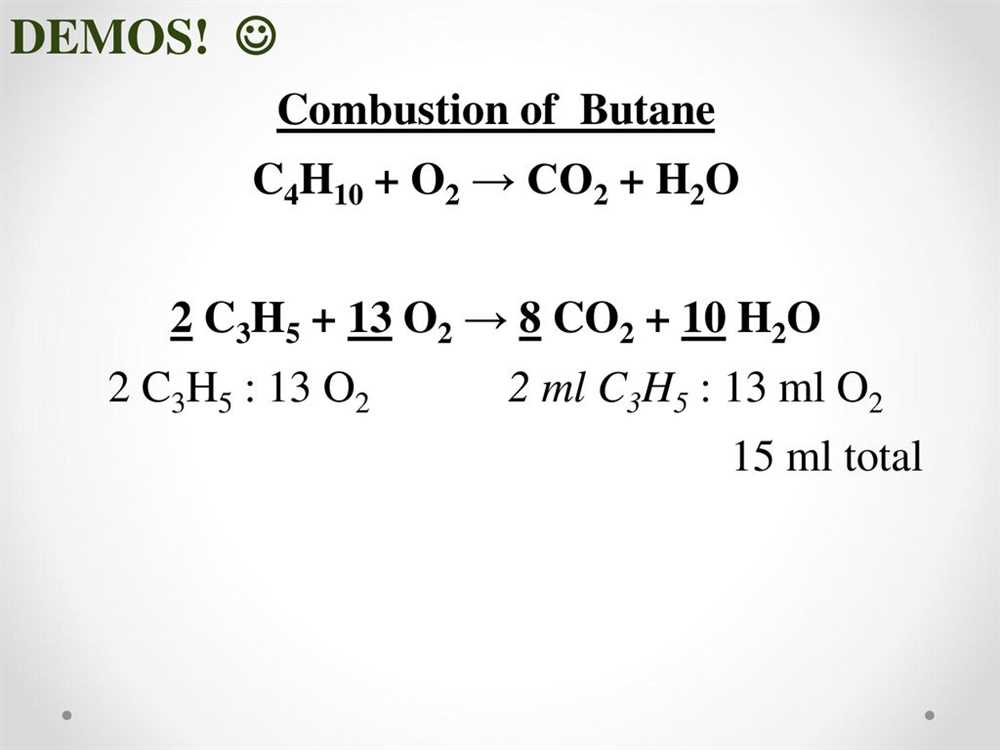
In chemistry, balancing chemical equations is an essential skill that allows us to understand and predict the reactions that occur during chemical processes. A balanced chemical equation shows the correct ratio of reactants and products involved in a chemical reaction.
Step 1: Identify the reactants and products
The first step in balancing a chemical equation is to identify the reactants and products. Reactants are the substances that undergo a chemical change, while products are the substances that are produced as a result of the reaction.
Step 2: Write the unbalanced equation
Once the reactants and products have been identified, write the unbalanced equation by placing the formulas of the reactants on the left side of the arrow and the formulas of the products on the right side of the arrow.
Step 3: Count the number of atoms
Count the number of atoms of each element on both sides of the equation. This will help you determine if the equation is balanced or not.
Step 4: Balance the equation
To balance the equation, adjust the coefficients in front of the formulas so that the number of atoms of each element is the same on both sides of the equation. The coefficients represent the relative amounts of each substance involved in the reaction.
Step 5: Check your work
After balancing the equation, double-check your work by counting the number of atoms of each element on both sides of the equation. If the number of atoms is the same on both sides, then the equation is balanced.
Example:
Let’s balance the equation: H2 + O2 -> H2O
Step 1: Identify the reactants and products – H2 and O2 are the reactants, and H2O is the product.
Step 2: Write the unbalanced equation – H2 + O2 -> H2O
Step 3: Count the number of atoms – 2 hydrogen atoms and 2 oxygen atoms on the left side, and 2 hydrogen atoms and 1 oxygen atom on the right side.
Step 4: Balance the equation – multiply the coefficients to achieve the same number of atoms on both sides: 2H2 + O2 -> 2H2O.
Step 5: Check your work – 4 hydrogen atoms and 2 oxygen atoms on both sides, so the equation is balanced.
By following these steps, you can successfully balance chemical equations and gain a deeper understanding of chemical reactions.
Understanding the Mole Ratio Concept
The mole ratio concept is a fundamental concept in stoichiometry, which is the study of the quantitative relationships between reactants and products in chemical reactions. In a chemical equation, the coefficients represent the number of moles of each substance involved in the reaction. These coefficients can be used to determine the mole ratio between different substances in the reaction.
A mole ratio is a conversion factor that relates the number of moles of one substance to the number of moles of another substance in a chemical reaction. It is obtained by dividing the coefficients of the two substances in the balanced chemical equation. For example, in the equation 2 H₂ + O₂ → 2 H₂O, the mole ratio between hydrogen and water is 2 moles of H₂ to 2 moles of H₂O.
The concept of mole ratio is crucial for performing stoichiometric calculations, such as determining the amount of product that can be formed from a given amount of reactants or vice versa. By using the mole ratio, it is possible to convert between different substances and quantities in a chemical equation.
The mole ratio concept is based on the principle of conservation of mass, which states that the total mass of the reactants must be equal to the total mass of the products in a chemical reaction. By using the mole ratio, it is possible to determine the exact amount of each substance that is involved in the reaction and predict the outcome of the reaction.
In conclusion, the mole ratio concept is a fundamental concept in stoichiometry that allows for the calculation of the amount of substances involved in a chemical reaction. Understanding this concept is essential for performing stoichiometric calculations and predicting the outcome of chemical reactions.
Calculating Stoichiometric Ratios
When completing stoichiometry calculations, it is essential to understand the concept of stoichiometric ratios. These ratios are determined by the balanced chemical equation for the reaction. They represent the relationship between the number of moles of reactants and products involved in the reaction.
To calculate stoichiometric ratios, first, determine the balanced chemical equation for the reaction. This equation shows the reactants on the left side and the products on the right side. Each compound or element is represented by its chemical formula, and the coefficients in front of the formulas indicate the relative number of moles of each substance in the reaction.
To determine the stoichiometric ratio between two substances, compare the coefficients of the reactant and the product in the balanced equation. For example, if the coefficient of reactant A is 2 and the coefficient of product B is 3, the stoichiometric ratio between A and B would be 2:3.
Stoichiometric ratios are crucial for performing calculations such as determining the limiting reactant, calculating the amount of product formed, or predicting the amount of excess reactant remaining. These calculations rely on the fact that reactants are consumed in proportion to their stoichiometric ratios, allowing one to determine the amounts of substances involved in the reaction.
Overall, calculating stoichiometric ratios is fundamental in stoichiometry as it helps in quantifying the relationship between reactants and products in a chemical reaction. By understanding these ratios, chemists can determine the amounts of substances involved in a reaction, aiding in experimental design and theoretical calculations.
Converting Between Grams, Moles, and Particles
Converting between grams, moles, and particles is an essential skill in stoichiometry. It allows us to compare different substances and determine the amounts of reactants and products in a chemical reaction. Understanding this conversion process is crucial for solving stoichiometry problems.
The conversion between grams, moles, and particles is based on the concept of molar mass, which is the mass of one mole of a substance. By using the molar mass, we can convert between grams and moles. For example, to convert grams to moles, we divide the given mass by the molar mass. On the other hand, to convert moles to grams, we multiply the given number of moles by the molar mass.
- To convert grams to moles: divide the given mass by the molar mass.
- To convert moles to grams: multiply the given number of moles by the molar mass.
In addition to grams and moles, we can also convert between moles and particles. Avogadro’s number is used to convert between these two units. Avogadro’s number represents the number of particles in one mole of a substance (6.022 x 10^23). To convert moles to particles, we multiply the given number of moles by Avogadro’s number. Conversely, to convert particles to moles, we divide the given number of particles by Avogadro’s number.
- To convert moles to particles: multiply the given number of moles by Avogadro’s number.
- To convert particles to moles: divide the given number of particles by Avogadro’s number.
Being able to convert between grams, moles, and particles allows us to determine the stoichiometry of a chemical reaction, such as the ratio of reactants and products. It is an essential skill in chemistry and is used in various calculations, such as determining the limiting reactant, percent yield, and empirical formula.
Limiting Reactants and Excess Reactants
In a chemical reaction, the limiting reactant is the reactant that is completely consumed first, thereby limiting the amount of product that can be formed. The excess reactant, on the other hand, is the reactant that is left over after the limiting reactant is completely consumed. Identifying the limiting reactant and excess reactant is crucial in determining the maximum amount of product that can be produced.
To determine the limiting reactant, one needs to compare the stoichiometric ratios between the reactants and the desired product. The reactant with the smallest ratio is the limiting reactant, as it will be completely consumed first, while the reactant with the larger ratio will be in excess. The amount of product formed is determined by the amount of limiting reactant present.
For example, consider the reaction between hydrogen and oxygen to form water:
- 2H2 + O2 → 2H2O
If you have 4 moles of hydrogen and 2 moles of oxygen, you can see that the ratio between hydrogen and oxygen is 2:1. This means that for every 2 moles of hydrogen, 1 mole of oxygen is needed. Since you have 2 moles of oxygen, it is the limiting reactant because you would need 4 moles of oxygen to react completely with the 4 moles of hydrogen. Therefore, hydrogen is the excess reactant in this case.
In conclusion, determining the limiting reactant and excess reactant is essential for understanding the stoichiometry of a chemical reaction. It allows us to calculate the maximum amount of product that can be obtained and the amount of excess reactant remaining. By understanding these concepts, chemists can optimize reaction conditions and minimize waste in industrial processes.