The study of the electromagnetic spectrum and light is a key component in understanding the properties of light and its interaction with matter. In Chapter 18, we delve deeper into these concepts and explore the different types of electromagnetic waves and their uses in various fields.
One of the main focuses of this chapter is understanding the relationship between wavelength, frequency, and energy. By examining the electromagnetic spectrum, which encompasses all the different types of electromagnetic waves, from radio waves to gamma rays, we can gain a better understanding of how these factors affect the behavior of light.
We also explore the properties of light and the various phenomena associated with it, such as reflection, refraction, and diffraction. By understanding these principles, we can explain why objects appear certain colors, why light bends when it passes through different mediums, and how lenses can manipulate light to form images.
This answer key provides a comprehensive breakdown of the key concepts covered in Chapter 18. It serves as a valuable resource for students and educators alike, allowing them to assess their understanding and reinforce their knowledge of the electromagnetic spectrum and light. With this key in hand, readers can deepen their understanding of light and its intriguing properties.
Chapter 18: The Electromagnetic Spectrum and Light Answer Key
In Chapter 18 of the textbook, we explore the fascinating world of the electromagnetic spectrum and uncover the mysteries of light. This answer key is designed to help you navigate through the chapter and check your understanding of the key concepts and principles discussed.
Section 1: The Nature of Light
In this section, we delve into the fundamental properties and characteristics of light. You will learn about the wave-particle duality of light, where light behaves both as a wave and as a particle called a photon. We will also discuss the speed of light, which is a universal constant in a vacuum, and how it differs when traveling through different mediums.
Section 2: The Electromagnetic Spectrum
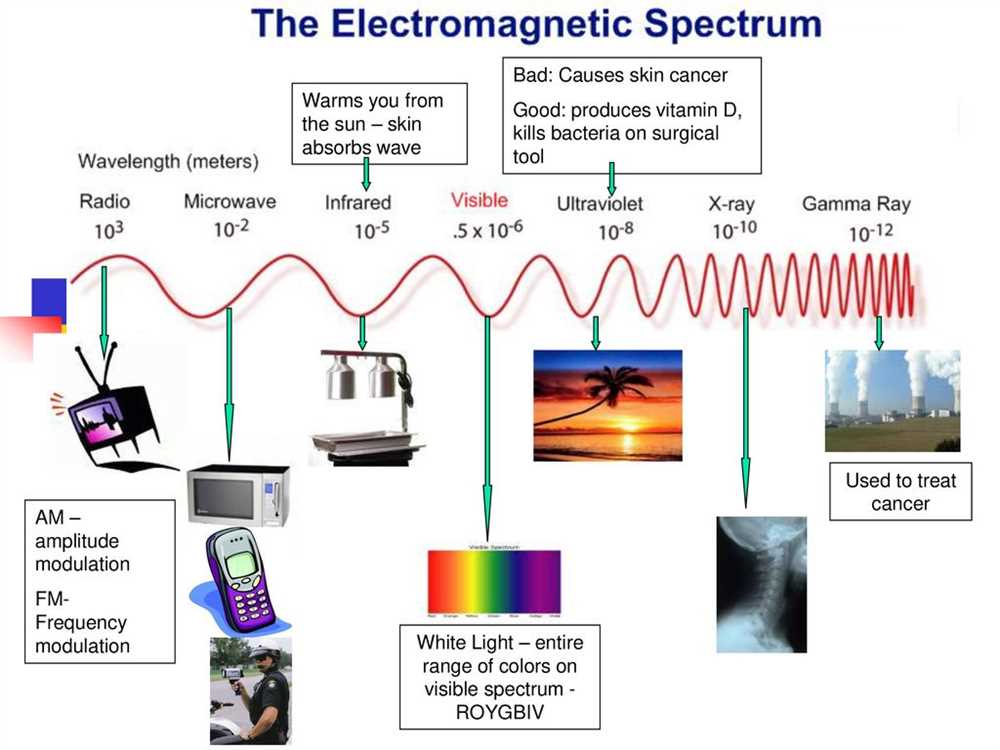
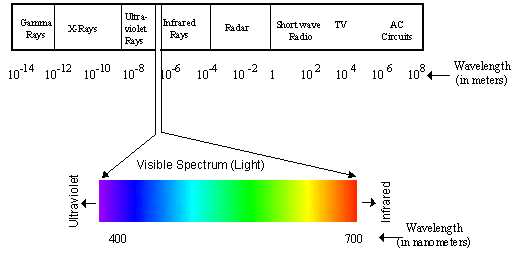
In Section 2, we explore the electromagnetic spectrum, which is a range of all possible frequencies of electromagnetic radiation. You will learn about the different types of electromagnetic waves, including radio waves, microwaves, infrared radiation, visible light, ultraviolet radiation, X-rays, and gamma rays. We will study their properties, applications, and how they interact with matter.
Section 3: The Behavior of Light
This section focuses on the behavior of light when it interacts with different types of objects. You will learn about reflection, refraction, diffraction, and interference of light. We will examine how these phenomena occur and how they can be applied in various fields, including optics, photography, and telecommunications.
Section 4: Light and Color
Section 4 delves into the fascinating world of color. You will learn about the properties of light that determine its color, such as its wavelength and frequency. We will discuss the additive and subtractive color models, color mixing, and the concept of complementary colors. We will also explore the physiology of vision and how our eyes perceive different colors.
Section 5: Applications of Light
In the final section of the chapter, we examine the numerous applications of light in various fields. You will learn about fiber optics, which revolutionized telecommunications and data transmission. We will discuss the use of light in medical imaging and photography and explore its applications in astronomy and remote sensing. We will also touch upon the emerging field of quantum optics and its potential for future technologies.
This answer key should serve as a valuable tool to check your understanding of the concepts covered in Chapter 18. Make sure to refer back to your textbook for detailed explanations and examples to reinforce your knowledge. Enjoy exploring the fascinating world of the electromagnetic spectrum and light!
Understanding the Electromagnetic Spectrum
The electromagnetic spectrum is a range of electromagnetic radiation that includes everything from radio waves to gamma rays. It is divided into different regions based on the wavelength or frequency of the electromagnetic waves. Each region has its own unique properties and interactions with matter. Understanding the electromagnetic spectrum is essential in various fields, including physics, astronomy, and telecommunications.
At one end of the spectrum, we have radio waves, which have the longest wavelengths and lowest frequencies. These waves are used for communication, such as broadcasting radio and television signals. Moving along the spectrum, we encounter microwaves, which are used for various purposes, including cooking food and transmitting data wirelessly.
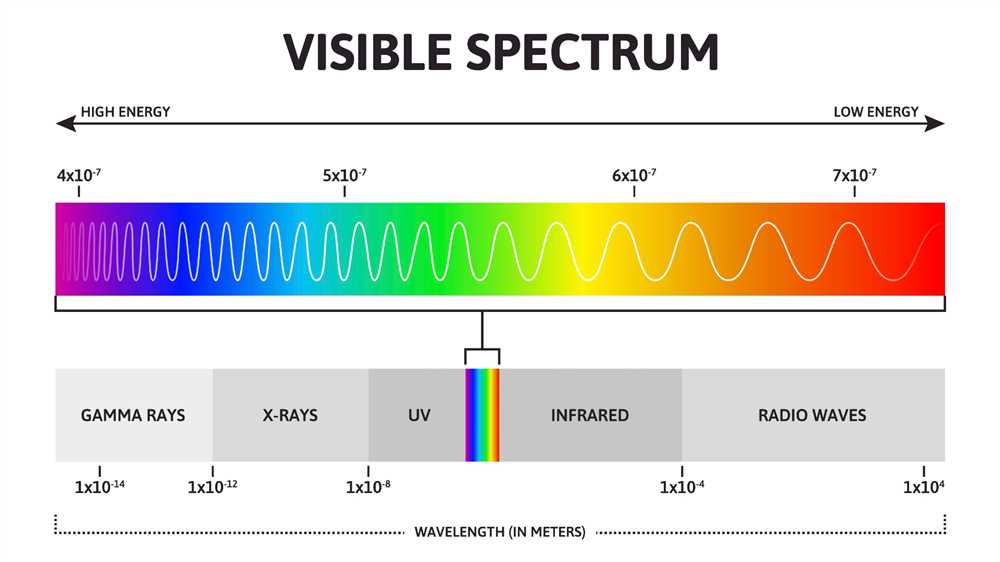
Next, we have infrared radiation, which is often associated with heat. Infrared waves are utilized in various applications, such as remote controls, night vision devices, and thermal imaging cameras. Beyond infrared, we find visible light, which is the only part of the spectrum that our eyes can perceive. The different colors of visible light correspond to different wavelengths. For example, red light has a longer wavelength than blue light.
Continuing further, we come across ultraviolet radiation, which is responsible for causing sunburns and is commonly used in sterilization processes. X-rays have shorter wavelengths and higher frequencies than ultraviolet radiation. They are widely used in medical imaging to visualize bones and internal organs. Finally, we have gamma rays, which have the shortest wavelengths and highest frequencies. These rays are produced during nuclear reactions and are used in cancer treatment and sterilization procedures.
In conclusion, the electromagnetic spectrum is a vast range of electromagnetic radiation that encompasses various forms of energy. Understanding the different regions of the spectrum and their applications is crucial for advancements in technology, communication, and scientific research.
Exploring the Different Types of Waves in the Electromagnetic Spectrum
The electromagnetic spectrum is a vast range of waves, each with varying wavelengths and frequencies. These waves include radio waves, microwaves, infrared waves, visible light, ultraviolet waves, X-rays, and gamma rays. Each type of wave is categorized based on its length and energy level.
Starting with radio waves, they have the longest wavelengths and lowest frequencies in the electromagnetic spectrum. They are commonly used for communication purposes, such as radio broadcasting and cell phone transmissions. Microwaves, on the other hand, have slightly shorter wavelengths and higher frequencies. They are used in various applications, including cooking, radar systems, and satellite communication.
Infrared waves have even shorter wavelengths and higher frequencies than microwaves. They are often associated with heat and are used in thermal imaging, remote controls, and certain medical procedures. Visible light, the only type of wave that can be detected by the human eye, falls in the middle of the electromagnetic spectrum. It consists of seven colors: red, orange, yellow, green, blue, indigo, and violet, each with a different wavelength and frequency.
Ultraviolet waves have shorter wavelengths and higher frequencies than visible light. They are known for their ability to cause sunburn and are used in sterilization processes. X-rays, with even shorter wavelengths and higher frequencies, are commonly used in medical imaging, such as X-ray machines and CT scans. Finally, gamma rays have the shortest wavelengths and highest frequencies in the electromagnetic spectrum. They are extremely powerful and are often used in cancer treatment and radiation therapy.
In summary, the electromagnetic spectrum encompasses a wide range of waves, each with its own characteristics and applications. Understanding and harnessing these waves have led to significant advancements in communication, technology, and medicine.

The Relationship Between Wavelength, Frequency, and Energy
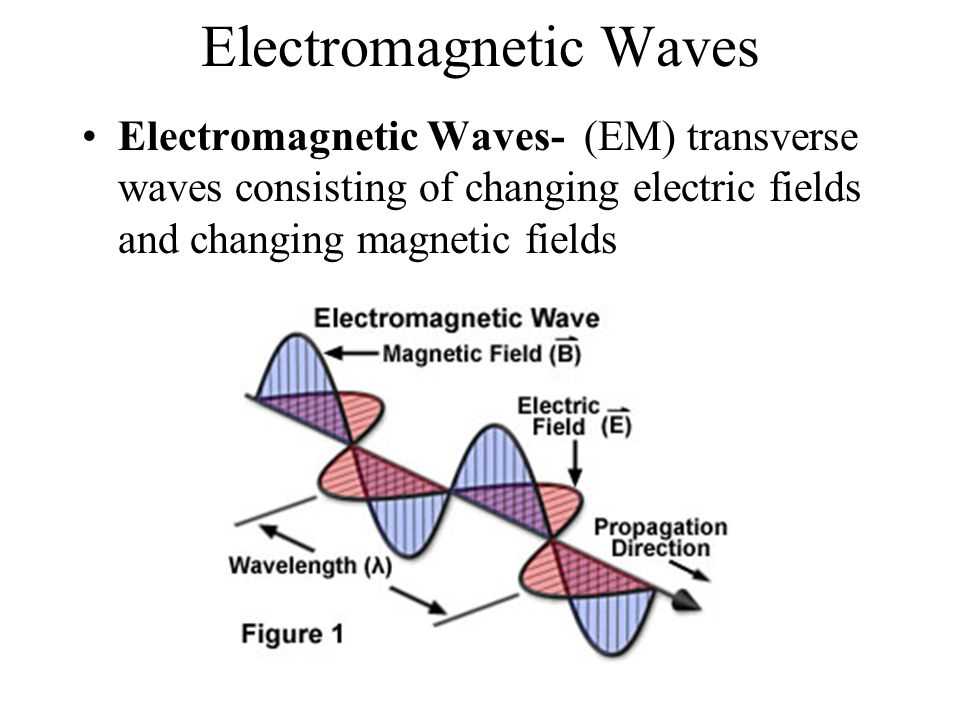
Understanding the relationship between wavelength, frequency, and energy is crucial in studying the electromagnetic spectrum and light. Each of these properties is interconnected and affects the behavior and characteristics of electromagnetic waves.
Wavelength refers to the distance between two consecutive peaks or troughs of a wave. It is usually represented by the symbol λ (lambda) and is measured in meters (m) or other units of length. The wavelength determines the type of electromagnetic wave, with shorter wavelengths corresponding to higher energy waves, and longer wavelengths corresponding to lower energy waves.
Frequency, on the other hand, refers to the number of wave cycles that pass a given point in a unit of time. It is usually represented by the symbol f and is measured in hertz (Hz) or other units of frequency. The frequency of a wave is inversely proportional to its wavelength, meaning that waves with shorter wavelengths have higher frequencies, and waves with longer wavelengths have lower frequencies.
The energy of an electromagnetic wave is directly proportional to its frequency. This means that waves with higher frequencies have more energy, while waves with lower frequencies have less energy. The energy of a wave is related to its amplitude, or the height of its peaks, but it is primarily determined by its frequency.
In conclusion, wavelength, frequency, and energy are all interconnected properties of electromagnetic waves. Understanding their relationship is essential in comprehending how different waves behave and interact with matter. It allows scientists to categorize waves in the electromagnetic spectrum and study their various applications in fields such as telecommunications, medicine, and astronomy.
Properties of Light and its Behavior
Light is a form of electromagnetic radiation that can be described as a wave or a particle. It has several properties that determine how it behaves and interacts with matter. One important property of light is its wavelength, which is the distance between two consecutive peaks or troughs in a wave. Wavelength is directly related to the color of light, with longer wavelengths corresponding to red light and shorter wavelengths corresponding to blue or violet light.
Another property of light is its frequency, which is the number of waves that pass a given point in a second. Frequency is inversely related to wavelength, meaning that as wavelength increases, frequency decreases, and vice versa. The speed of light is constant in a vacuum, meaning that the product of wavelength and frequency is always equal to the speed of light, which is approximately 3.00 x 10^8 meters per second.
Light also exhibits the property of reflection, which occurs when light waves bounce off a surface. The angle of incidence, which is the angle at which light hits a surface, is equal to the angle of reflection, which is the angle at which light bounces off the surface. This property allows us to see objects that do not emit light themselves, such as the moon or a mirror. Refraction is another behavior of light, which occurs when light waves pass through a medium and change direction. This can be observed when light passes through a prism and separates into its component colors.
In addition to reflection and refraction, light can also be absorbed and transmitted. When light is absorbed, it is converted into another form of energy, such as heat. When light is transmitted, it passes through a transparent or translucent material without being absorbed or reflected. These behaviors of light allow us to manipulate it using lenses, mirrors, and filters, and they play a crucial role in many technological applications, such as photography, fiber optics, and laser technology.
Interaction of Light with Matter
Light is an electromagnetic wave that can interact with matter in various ways, leading to a wide range of phenomena and applications. When light interacts with matter, it can be absorbed, transmitted, reflected, or refracted, depending on the properties of both the light and the material it interacts with.
Absorption: When light is absorbed by a material, its energy is transferred to the atoms or molecules of the material, causing them to vibrate or move. This can result in the heating of the material or the excitation of its electrons to higher energy levels. The absorbed energy can also lead to chemical reactions or other changes in the material.
Transmission: If light passes through a material without being absorbed or scattered, it is said to be transmitted. Transparent materials, such as glass or air, allow light to pass through them with minimal absorption. The ability of a material to transmit light depends on its structure and optical properties, such as its refractive index.
Reflection: When light strikes a surface, it can be reflected, bouncing off the surface in a different direction. The angle at which the light is reflected depends on the angle at which it strikes the surface and the properties of the material. Mirrors, for example, are designed to have a smooth surface that reflects light in a predictable manner.
Refraction: Refraction occurs when light passes from one medium to another, causing it to change direction. This change in direction is due to the difference in optical density between the two media. The degree of refraction depends on the angle at which the light enters the second medium and the refractive indices of both the first and second media. This phenomenon is responsible for the bending of light when it passes through a lens.
Scattering: When light interacts with small particles or irregularities in a material, it can be scattered in different directions. This is why the sky appears blue during the day, as the shorter blue wavelengths are scattered more than the longer red wavelengths. Scattering can also occur in gases, liquids, or solids, and it plays a crucial role in various natural phenomena, such as the formation of rainbows.
Overall, the interaction of light with matter is a complex and fascinating subject that has implications in various fields, including optics, materials science, and biology. Understanding how light behaves when it interacts with different materials is essential for the development of new technologies and for gaining insights into the fundamental nature of matter and energy.