
In Chapter 2 of our biology textbook, we delved into the fascinating world of chemical reactions and enzymes. This lesson, Lesson 4, provides the answer key to test your understanding and knowledge of this crucial topic. Understanding chemical reactions and how enzymes function is essential for studying the intricacies of biological systems and processes.
Chemical reactions are the foundation of life, occurring within every living organism. They involve the breaking and forming of bonds between atoms, resulting in the rearrangement of atoms to create new substances. Enzymes, on the other hand, are highly specialized proteins that act as catalysts, facilitating and speeding up chemical reactions. Without enzymes, these reactions would occur too slowly to sustain life.
In Lesson 4, we explored the factors that affect the rate of chemical reactions and how enzymes function. Topics covered include the role of activation energy, the importance of temperature and pH, and the specificity and efficiency of enzymes. This answer key serves as a valuable resource to gauge your understanding of these concepts and reinforce your learning.
Chapter 2 Lesson 4 Chemical Reactions and Enzymes Answer Key

In this lesson, we will explore the concept of chemical reactions and enzymes. Chemical reactions are processes where substances, called reactants, interact with each other to form new substances, known as products. These reactions involve the breaking and making of bonds between atoms, which results in the rearrangement of atoms to form different molecules.
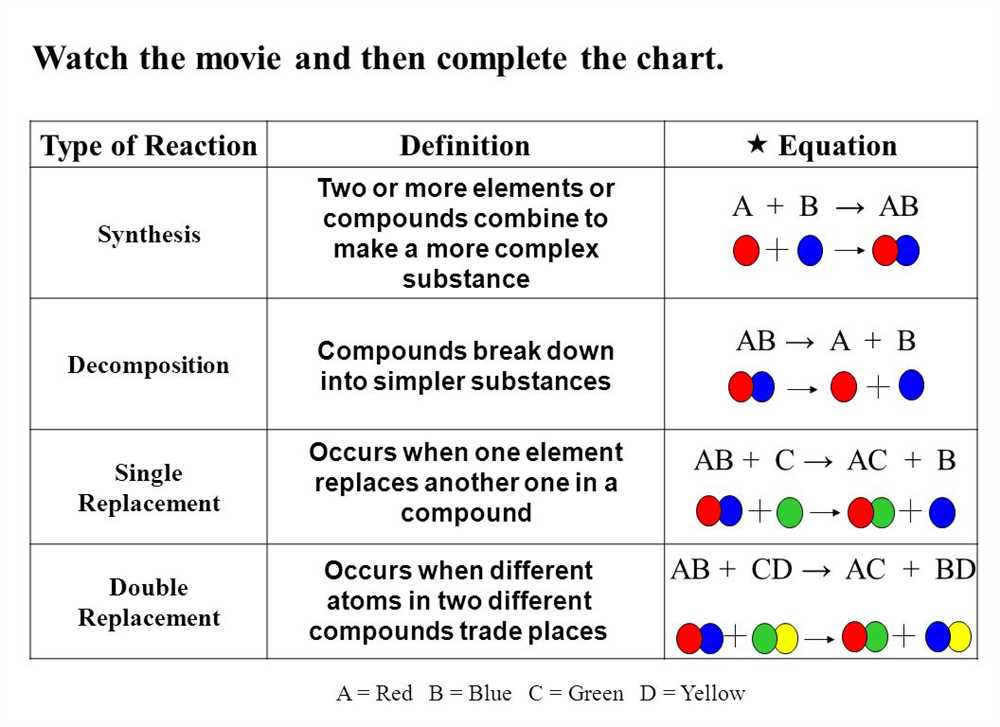
One important type of chemical reaction is the synthesis reaction, where two or more reactants combine to form a single product. This can be represented by the general equation: A + B → AB. Another type of reaction is the decomposition reaction, where a single reactant breaks down into two or more products. This can be represented by the general equation: AB → A + B.
Enzymes are biological catalysts that speed up the rate of chemical reactions in living organisms. They are usually proteins that bind to specific substrates and help facilitate the conversion of reactants into products. Enzymes lower the activation energy required for a reaction to occur, making it easier for the reaction to proceed. Additionally, enzymes are highly specific and can only catalyze a particular reaction or a group of structurally similar reactions.
To sum up, chemical reactions involve the breaking and making of bonds between atoms to form new substances. Enzymes are biological catalysts that speed up these reactions by reducing the activation energy. Understanding the key concepts of chemical reactions and enzymes is crucial in studying and comprehending various biological and chemical processes.
Understanding Chemical Reactions
In the world of chemistry, chemical reactions play a crucial role. Understanding these reactions is essential for scientists and researchers to develop new materials, drugs, and processes. Chemical reactions involve the transformation of one or more substances into different substances, accompanied by the breaking and formation of chemical bonds.
Types of Chemical Reactions:
There are various types of chemical reactions that occur based on the changes in the atoms and molecules. Some common types include:
- Combination Reactions: These reactions involve the combination of two or more substances to form a single product. For example, the reaction between hydrogen and oxygen to form water (2H₂ + O₂ → 2H₂O).
- Decomposition Reactions: In these reactions, a single compound breaks down into simpler substances. This can be represented by the equation AB → A + B. An example is the decomposition of hydrogen peroxide into water and oxygen (2H₂O₂ → 2H₂O + O₂).
- Single Displacement Reactions: In these reactions, one element replaces another element in a compound. The general equation for this type of reaction is A + BC → AC + B. An example is the reaction between zinc and hydrochloric acid to form zinc chloride and hydrogen gas (Zn + 2HCl → ZnCl₂ + H₂).
- Double Displacement Reactions: These reactions involve the exchange of ions between two compounds. The general equation is AB + CD → AD + CB. For instance, the reaction between sodium chloride and silver nitrate to form sodium nitrate and silver chloride (NaCl + AgNO₃ → NaNO₃ + AgCl).
Enzymes in Chemical Reactions:
Enzymes are biological catalysts that speed up chemical reactions in living organisms. They play a crucial role in various metabolic processes, such as digestion, respiration, and energy production. Enzymes work by lowering the activation energy required for a reaction to occur, allowing it to happen more rapidly. They are highly specific and can recognize and bind to specific substrates to catalyze the desired reaction.
Understanding chemical reactions is fundamental to the field of chemistry. By studying the various types of reactions and the role of enzymes, scientists can further our understanding of the natural world and develop new technologies to improve our lives.
Key Concepts in Chemical Reactions
Chemical reactions are fundamental processes in nature that involve the transformation of one or more substances into different substances. Understanding the key concepts in chemical reactions is essential for comprehending the intricacies of how matter and energy interact and change.
Reactants and products: In a chemical reaction, reactants are the starting substances that undergo a chemical change, while products are the resulting substances formed after the reaction. Reactants and products are represented by chemical equations, which show the chemical formulas of the substances involved.
Conservation of mass: The law of conservation of mass states that in a chemical reaction, the total mass of the reactants is equal to the total mass of the products. This means that matter cannot be created or destroyed during a chemical reaction, only rearranged.
- Types of reactions: Chemical reactions can be classified into several types based on the nature of the reaction. These include combination reactions, decomposition reactions, displacement reactions, and redox reactions.
- Reaction rates: The rate of a chemical reaction refers to the speed at which reactants are transformed into products. Various factors can affect the rate of a reaction, such as temperature, concentration, and the presence of catalysts.
- Enzymes: Enzymes are biological catalysts that increase the rate of chemical reactions in living organisms. They are highly specific and can catalyze specific reactions without being consumed in the process.
Understanding these key concepts in chemical reactions provides a foundation for exploring the diverse aspects of chemistry, from understanding the behavior of substances to developing new materials and drugs. Chemical reactions play a crucial role in various scientific fields and industries, making them a fascinating and essential area of study.
The Role of Enzymes in Chemical Reactions
Enzymes play a vital role in facilitating chemical reactions within living organisms. These specialized proteins act as catalysts, speeding up the rate of a reaction without being consumed in the process. Through their unique structure and mechanism, enzymes are able to lower the activation energy required for a reaction to occur, making it easier for the reaction to proceed.
Enzymes are highly specific in their function and can only catalyze a particular reaction or a group of closely related reactions. This specificity is attributed to the active site of the enzyme, which is a small region where the substrate molecules bind and undergo a chemical transformation. The active site has a three-dimensional shape that is complementary to the shape of the substrate, allowing for precise recognition and binding.
When a substrate molecule enters the active site of an enzyme, it undergoes a series of interactions that result in the formation of an enzyme-substrate complex. Within this complex, the enzyme modifies the substrate molecule, either by breaking it apart or combining it with other molecules, to form the desired products. Once the reaction is complete, the products are released, and the enzyme can be reused to catalyze additional reactions.
Enzymes are highly efficient in their catalytic function, often increasing the rate of a reaction by several orders of magnitude. They accomplish this by stabilizing reaction intermediates, orienting substrates in a favorable position, and providing functional groups that participate in the reaction. The presence of enzymes allows organisms to carry out essential metabolic processes at temperatures and concentrations that would otherwise be too slow or energetically unfavorable.
- Enzymes play a crucial role in chemical reactions by acting as catalysts.
- Their unique structure and mechanism lower the activation energy required for a reaction.
- Enzymes are highly specific and catalyze specific reactions or groups of closely related reactions.
- The active site of the enzyme binds and transforms substrate molecules.
- Enzymes are highly efficient, increasing the rate of reactions by several orders of magnitude.
Exploring the Process of Enzyme Catalysis
Enzymes play a crucial role in the chemical reactions that occur within living organisms. They act as catalysts, speeding up these reactions by lowering the activation energy required for a reaction to occur. This allows the reactions to take place at a faster rate, enabling the necessary biological processes to happen efficiently.
An enzyme-catalyzed reaction typically follows a specific process. First, the enzyme and the substrate, or the molecule on which the enzyme acts, come together to form an enzyme-substrate complex. This occurs when the substrate binds to the active site of the enzyme, which is a specific region that allows the enzyme to interact with the substrate.
Once the enzyme and substrate are bound together, the enzyme catalyzes the conversion of the substrate into the desired product or products. This conversion involves the breaking of chemical bonds within the substrate and the formation of new bonds to create the final products. The enzyme itself remains unchanged throughout the reaction and can be used again to catalyze similar reactions.
The effectiveness of an enzyme in catalyzing a reaction depends on various factors, including temperature, pH level, and substrate concentration. Enzymes have optimal conditions in which they function most efficiently, and deviations from these conditions can affect their catalytic activity. For example, extreme temperatures or pH levels can denature the enzyme and render it ineffective.
In conclusion, the process of enzyme catalysis involves the binding of the enzyme and substrate, the catalyzation of the reaction, and the formation of the final products. Understanding this process is essential for studying and manipulating biological systems, as it provides insight into how enzymes contribute to various biochemical processes in living organisms.
Factors Affecting Enzyme Activity

Enzymes are biological molecules that accelerate chemical reactions in living organisms. They play a crucial role in various physiological processes, such as metabolism, cellular signaling, and digestion. However, the activity of enzymes can be influenced by several factors, which can either enhance or inhibit their function.
Temperature: Enzymes have an optimal temperature at which they work most efficiently. This temperature varies depending on the enzyme, but it is typically around the normal body temperature of 37°C for human enzymes. When the temperature deviates from the optimal range, the enzyme’s activity decreases. At low temperatures, the enzyme may become inactive, while at high temperatures, it may denature and lose its structure and function.
pH: Enzymes also have an optimal pH at which they function best. Each enzyme has a specific pH range in which it can catalyze reactions effectively. Deviation from this optimal pH can impair enzyme activity. For example, stomach enzymes work in an acidic environment, such as the one found in the stomach, while intestinal enzymes require a more alkaline pH.
Substrate Concentration: The rate of enzyme activity is usually proportional to substrate concentration up until a point of saturation. An increase in substrate concentration leads to more frequent collisions between the enzyme and substrate molecules, resulting in an increased rate of reaction. However, once the enzyme is saturated with substrate, the reaction rate reaches its maximum, and further increase in substrate concentration has no effect on the rate of reaction.
Inhibitors: Enzyme activity can be inhibited by various substances, known as inhibitors. Inhibitors can be either competitive or non-competitive. Competitive inhibitors compete with the substrate for the active site of the enzyme, thereby blocking the binding of the substrate. Non-competitive inhibitors, on the other hand, bind to a different site on the enzyme, causing a change in the enzyme’s structure and rendering it inactive.
Understanding the factors that affect enzyme activity is crucial in various fields, including medicine, agriculture, and biotechnology. By manipulating these factors, scientists can optimize the activity of enzymes or regulate their function, leading to potential applications in drug development, food production, and environmental remediation.
Enzyme Inhibition and Regulation
Enzymes play a crucial role in catalyzing chemical reactions within cells. However, their activity needs to be carefully regulated to maintain the proper functioning of biological processes. One way that enzyme activity can be regulated is through enzyme inhibition. Inhibition occurs when a molecule binds to an enzyme and prevents it from carrying out its normal function.
There are two main types of enzyme inhibition: competitive inhibition and non-competitive inhibition. In competitive inhibition, the inhibitor molecule competes with the substrate for binding to the active site of the enzyme. This prevents the substrate from binding and the enzyme from catalyzing the reaction. Non-competitive inhibition, on the other hand, occurs when the inhibitor binds to a different site on the enzyme, known as the allosteric site. This changes the shape of the enzyme, making it unable to bind to the substrate and carry out the reaction.
Competitive inhibition is reversible, meaning that the inhibitor can dissociate from the enzyme and the enzyme can regain its activity. This type of inhibition can be overcome by increasing the concentration of the substrate, as this increases the chances of the substrate binding to the active site instead of the inhibitor.
Non-competitive inhibition, on the other hand, is often irreversible. The inhibitor molecule permanently binds to the allosteric site, rendering the enzyme inactive. This type of inhibition cannot be overcome by increasing the substrate concentration, as the inhibitor does not compete with the substrate for binding.
In addition to inhibition, enzyme activity can also be regulated through various other mechanisms such as feedback inhibition, where the end product of a metabolic pathway acts as an inhibitor for an enzyme earlier in the pathway. This helps maintain homeostasis by preventing the overproduction of certain substances.
Understanding enzyme inhibition and regulation is crucial for studying and developing drugs that target specific enzymes. By selectively inhibiting or activating enzymes, researchers can manipulate biochemical pathways and potentially treat various diseases.