Oxidation-reduction (redox) reactions are a fundamental concept in chemistry that involve the transfer of electrons between atoms or ions. Mastery of this topic is crucial for understanding many aspects of chemical reactions, including balancing equations, predicting products, and understanding the behavior of substances in various environments.
Chapter 20 of your chemistry textbook focuses on oxidation-reduction reactions and provides a comprehensive understanding of the key concepts and principles. In this chapter, you will learn how to identify oxidation and reduction reactions, assign oxidation numbers to elements, balance redox equations, and utilize the half-reaction method to determine the overall balanced equation.
This answer key serves as a valuable resource to check your understanding and verify your solutions for the exercises and problems presented in Chapter 20. It provides step-by-step solutions and explanations for each question, allowing you to assess your progress and identify any areas that may require further review and practice.
Whether you are studying for an exam, completing homework assignments, or simply seeking a deeper understanding of oxidation-reduction reactions, this answer key will prove to be an invaluable tool. With its comprehensive and detailed explanations, it aims to enhance your learning experience and help you develop a solid foundation in redox chemistry.
Chapter 20 Oxidation Reduction Reactions Answer Key
Oxidation reduction reactions, also known as redox reactions, are a fundamental concept in chemistry. In these reactions, there is a transfer of electrons between species. The species that loses electrons is said to be oxidized, while the species that gains electrons is said to be reduced.
The answer key for Chapter 20 of a textbook on oxidation reduction reactions provides a comprehensive guide to solving problems and understanding concepts related to redox reactions. It includes step-by-step explanations of the solutions to various problems, as well as important tips and strategies for approaching different types of questions.
The answer key includes a list of key terms and definitions related to oxidation reduction reactions, such as oxidizing agent, reducing agent, half-reaction, and oxidation number. This allows students to review and reinforce their understanding of these important concepts.
Additionally, the answer key provides examples of balanced redox equations and explains how to determine the oxidized and reduced species. It also covers topics such as balancing redox reactions in acidic and basic solutions, calculating oxidation numbers, and identifying redox reactions in everyday life.
Overall, the Chapter 20 oxidation reduction reactions answer key is an invaluable resource for students studying redox reactions. It helps them develop a deeper understanding of the subject and provides a solid foundation for further study in chemistry.
Understanding Oxidation and Reduction
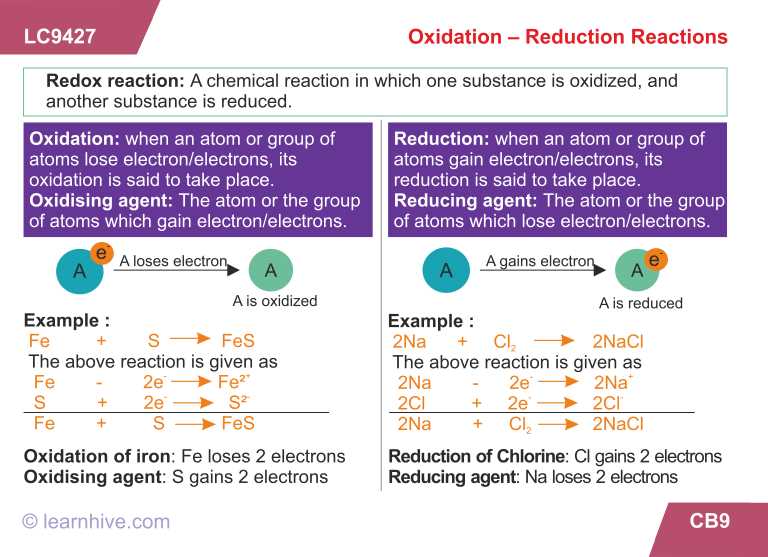
Oxidation and reduction are fundamental concepts in chemistry that involve the transfer of electrons between atoms or molecules. In an oxidation-reduction (redox) reaction, one species loses electrons and is oxidized, while another species gains electrons and is reduced. These reactions play a crucial role in many chemical processes, including combustion, corrosion, and biological reactions.
Oxidation is the process in which an atom or molecule loses electrons. It results in an increase in the oxidation state of that species. Reduction, on the other hand, is the process in which an atom or molecule gains electrons, leading to a decrease in its oxidation state. Oxidation and reduction always occur together in a redox reaction.
One way to determine whether a species is being oxidized or reduced is by examining its change in oxidation state. The oxidation state is a measure of the number of electrons that an atom has gained or lost compared to its neutral state. If the oxidation state of an atom increases in a reaction, it has been oxidized. Conversely, if the oxidation state decreases, the atom has been reduced.
In redox reactions, electrons are transferred from the species being oxidized (the reducing agent) to the species being reduced (the oxidizing agent). The reducing agent is oxidized and loses electrons, while the oxidizing agent is reduced and gains electrons. This transfer of electrons allows for the conversion of chemical energy into electrical energy, as seen in battery technologies.
Overall, understanding oxidation and reduction is crucial in chemistry as it helps explain the behavior of various substances in different chemical reactions. By identifying the oxidizing and reducing agents, as well as understanding the change in oxidation states, scientists can analyze and predict the outcome of redox reactions in various contexts.
Identifying Oxidation and Reduction
Oxidation-reduction (redox) reactions involve the transfer of electrons between reactants. In any redox reaction, there must be an element that is oxidized (loses electrons) and an element that is reduced (gains electrons). Identifying which species is oxidized and which is reduced is essential in understanding the overall reaction.
One way to identify oxidation and reduction is by analyzing changes in oxidation states. The oxidation state of an element is the hypothetical charge it would have if all its bonds were 100% ionic. Oxidation involves an increase in oxidation state, while reduction involves a decrease in oxidation state.
When analyzing a redox reaction, it is helpful to break it down into half-reactions. Each half-reaction represents either oxidation or reduction. In the oxidation half-reaction, the species that is being oxidized will have its oxidation state increase. In the reduction half-reaction, the species that is being reduced will have its oxidation state decrease.
A useful mnemonic device to remember oxidation and reduction is “LEO the lion says GER.” LEO stands for “lose electrons, oxidation” and GER stands for “gain electrons, reduction.” This serves as a reminder that oxidation is the loss of electrons, while reduction is the gain of electrons.
Identifying oxidation and reduction in a redox reaction allows us to balance the equation, calculate the oxidation state changes, and determine the overall change in electron transfer. It also helps in understanding the chemistry involved and predicting the behavior of substances in various reactions.
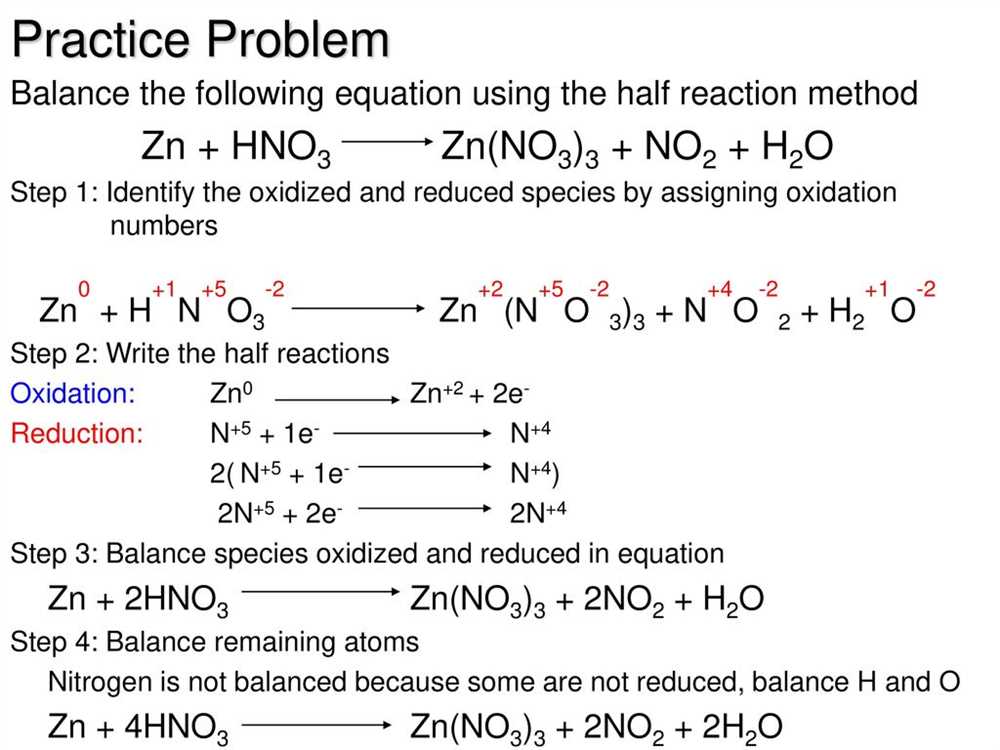
Balancing Oxidation Reduction Reactions
In chemical reactions, substances can undergo oxidation and reduction processes. These reactions are known as oxidation reduction reactions, or redox reactions. Balancing these reactions involves ensuring that the number of electrons gained during reduction is equal to the number of electrons lost during oxidation, as well as balancing the number of atoms on each side of the equation.
One method for balancing redox reactions is the half-reaction method. In this method, the reaction is split into two half-reactions: one for the oxidation process and one for the reduction process. Each half-reaction is balanced separately by adjusting the number of atoms and charges on each side of the equation.
The next step is to balance the number of electrons lost and gained in each half-reaction. The electrons lost in the oxidation half-reaction must be equal to the electrons gained in the reduction half-reaction. This is achieved by multiplying each half-reaction by a suitable coefficient that will balance the number of electrons.
Once the half-reactions are balanced, they can be combined to form the overall balanced equation. The electrons on both sides of the equation can be canceled out, leaving only the balanced atoms and charges. It is important to verify that the equation is balanced in terms of both mass and charge.
In summary, balancing oxidation reduction reactions involves splitting the reaction into half-reactions, balancing the atoms and charges in each half-reaction, and then balancing the electrons by adjusting the coefficients. The balanced half-reactions are then combined to form the overall balanced equation. Practice and understanding of the rules of oxidation numbers and balancing equations are essential for successfully balancing redox reactions.
Writing Half-Reactions
Writing half-reactions is an important skill in understanding and balancing oxidation-reduction reactions. Half-reactions are equations that represent the transfer of electrons during a redox reaction. They show the oxidation and reduction processes separately, making it easier to understand the overall reaction.
To write a half-reaction, it is important to identify the species that is being oxidized and the species that is being reduced. The species that loses electrons is oxidized, while the species that gains electrons is reduced. The half-reactions are then balanced by adding the appropriate number of electrons to each side of the equation.
For example, let’s consider the reaction between zinc and hydrochloric acid. In this reaction, zinc is oxidized to Zn2+ ions, while hydrogen ions are reduced to hydrogen gas. The half-reaction for the oxidation of zinc can be written as:
Zn(s) → Zn2+(aq) + 2e-
And the half-reaction for the reduction of hydrogen ions can be written as:
2H+(aq) + 2e- → H2(g)
These two half-reactions can then be combined to form the overall balanced equation for the reaction:
Zn(s) + 2H+(aq) → Zn2+(aq) + H2(g)
In this way, writing and balancing half-reactions allows us to better understand and analyze oxidation-reduction reactions.
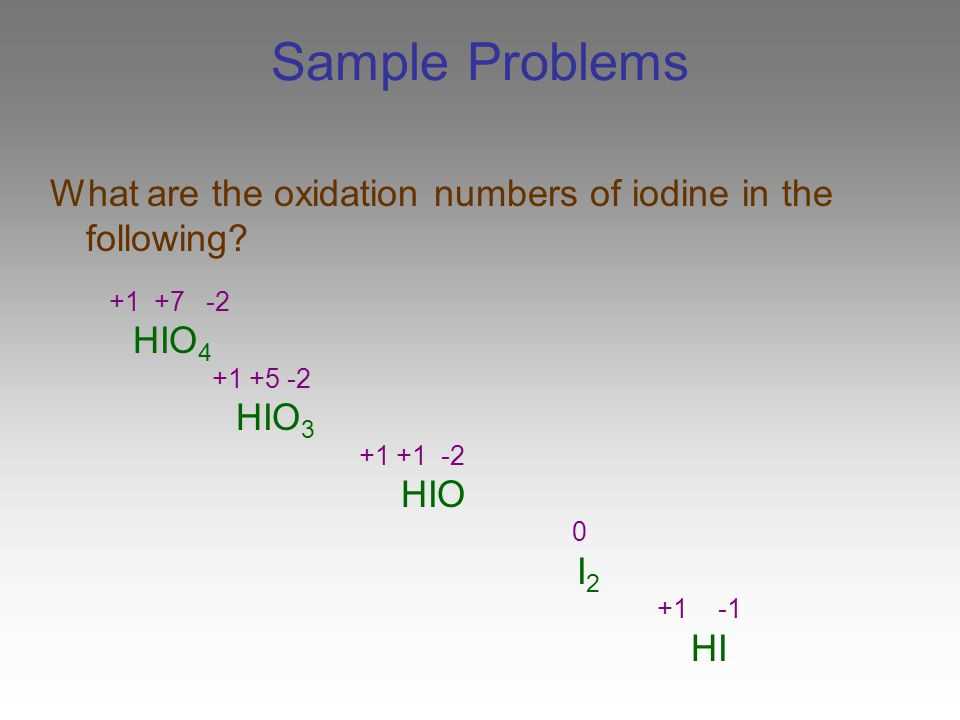
Determining Oxidation States
The oxidation state of an atom in a chemical compound is a way of representing the electron distribution and the relative positive or negative charge of that atom. It is a crucial concept in redox reactions, as it allows us to determine the degree of oxidation or reduction of the elements involved.
To determine the oxidation state of an atom in a compound, several guidelines can be followed:
- Rule 1: The oxidation state of an atom in its elemental state is always zero. For example, the oxidation state of oxygen in O2 is zero.
- Rule 2: In most compounds, Group 1A metals (except hydrogen) have an oxidation state of +1, and Group 2A metals have an oxidation state of +2.
- Rule 3: Hydrogen typically has an oxidation state of +1, but in binary compounds with less electronegative elements, it has an oxidation state of -1.
- Rule 4: Oxygen usually has an oxidation state of -2, except in peroxide compounds where it has an oxidation state of -1.
- Rule 5: The sum of the oxidation states of all the atoms in a compound is equal to the overall charge of the compound. For example, in NaCl, the sum of the oxidation state of Na (+1) and Cl (-1) is zero, reflecting the neutral charge of the compound.
By following these rules and considering the overall charge of the compound, it is possible to assign oxidation states to atoms in a chemical compound. This information is crucial for understanding the electron transfer and redox processes that occur in oxidation-reduction reactions.
Redox Reactions in Solution
In chemistry, redox reactions, also known as oxidation-reduction reactions, play a crucial role in both organic and inorganic chemistry. These reactions involve the transfer of electrons between species, resulting in changes in oxidation states.
Redox reactions in solution are commonly observed in various chemical processes, such as corrosion, electrolysis, and combustion. These reactions often involve the interaction between an oxidizing agent and a reducing agent. The oxidizing agent accepts electrons, while the reducing agent donates electrons.
One example of a redox reaction in solution is the oxidation of iron in the presence of oxygen and water, which leads to the formation of rust. In this reaction, iron undergoes oxidation, losing electrons to form Fe3+ ions, while oxygen is reduced, gaining electrons to form hydroxide ions.
Another important concept in redox reactions is the balancing of equations using the half-reaction method. This method involves breaking the reaction into two half-reactions, one representing oxidation and the other reduction. By balancing the number of electrons gained and lost in each half-reaction, the overall equation can be balanced.
In summary, redox reactions in solution involve the transfer of electrons between species, resulting in changes in oxidation states. These reactions play a crucial role in various chemical processes and can be balanced using the half-reaction method.
Electrochemical Cells
An electrochemical cell is a device that converts chemical energy into electrical energy, or vice versa. It consists of two half-cells, each containing an electrode immersed in an electrolyte solution. The two half-cells are connected by a salt bridge or a porous barrier that allows ion transfer between them.
At the heart of an electrochemical cell are the redox reactions that occur at the electrodes. In the oxidation half-cell, the electrode undergoes oxidation, losing electrons and generating positive ions in the solution. In the reduction half-cell, the electrode undergoes reduction, gaining electrons and producing negative ions in the solution. These half-cell reactions are governed by the principles of oxidation-reduction (redox) reactions.
The potential difference between the two electrodes, known as the cell voltage or electromotive force (EMF), determines the direction and magnitude of the electron flow. A positive cell voltage indicates a spontaneous reaction, where electrons flow from the anode (the negative electrode) to the cathode (the positive electrode). A negative cell voltage indicates a non-spontaneous reaction, requiring an external power source to force the electrons in the opposite direction.
Electrochemical cells have various applications in everyday life, such as in batteries, fuel cells, and electrolysis processes. Batteries use electrochemical cells to store and release electrical energy, while fuel cells convert chemical energy directly into electrical energy. Electrolysis processes use electrochemical cells to drive non-spontaneous reactions, such as the production of metals and the decomposition of water into hydrogen and oxygen gases.
In summary, electrochemical cells play a crucial role in the conversion of chemical energy into electrical energy and vice versa. They rely on redox reactions occurring at the electrodes to generate an electric current. Understanding the principles of electrochemical cells is essential for various technological advancements and applications.