
The study of atomic structure is essential in understanding the nature of matter. In Chapter 4, we delve into the intricate details of the composition of atoms and their constituents. This answer key serves as a valuable resource for students to check their understanding and verify their answers to the questions posed in this chapter.
One of the fundamental questions addressed in this chapter is the nature of the atomic nucleus. By examining the experiments conducted by early scientists, such as Ernest Rutherford’s gold foil experiment, we gain insight into the central role that protons and neutrons play in the atomic structure. Understanding the distribution and arrangement of these subatomic particles provides a foundation for comprehending the properties and behavior of atoms.
Additionally, this answer key explores the concept of atomic number and atomic mass. By connecting the number of protons in an atom to its unique identity, we can distinguish one element from another. Moreover, the discussion on isotopes sheds light on the existence of atoms with the same atomic number but different atomic masses. This understanding aids in interpreting the complexities of chemical reactions and the formation of compounds.
As we explore the answers to the questions in this chapter, we will uncover the intricate and fascinating world of atomic structure. Through the analysis of atomic models, the discovery of electrons, and the subsequent developments in quantum mechanics, we begin to grasp the complexity and beauty of the building blocks of matter. This answer key provides students with the opportunity to strengthen their knowledge and consolidate their understanding of the structure of the atom.
Chapter 4: The Structure of the Atom Answer Key
In Chapter 4 of our study on the structure of the atom, we delve into the answer key that unlocks the mysteries of atomic structure. By understanding the key concepts and principles outlined in this chapter, we can decipher the inner workings of atoms and gain a deeper understanding of the building blocks of matter.
One of the fundamental concepts covered in this chapter is the idea of atoms being composed of subatomic particles, namely protons, neutrons, and electrons. We learn that protons carry a positive charge, neutrons have no charge, and electrons carry a negative charge. These particles are organized in specific arrangements within an atom, giving it its unique properties.
Key Points:
- The atomic number of an atom is determined by the number of protons it contains.
- The mass number of an atom is the sum of its protons and neutrons.
- Isotopes are atoms of the same element that have different numbers of neutrons.
- The electron configuration of an atom determines its chemical behavior and reactivity.
- The periodic table provides a systematic arrangement of all known elements based on their atomic number and electron configuration.
By examining these key points, we can begin to unravel the intricate structure of atoms and understand how they interact with one another to form compounds and molecules. The answer key to Chapter 4 provides us with the necessary tools to navigate this world of atomic structure and paves the way for further exploration in the field of chemistry.
What is an Atom?
An atom is the smallest unit of matter that retains the characteristics of an element. Atoms are composed of three main subatomic particles: protons, neutrons, and electrons. Protons have a positive charge, neutrons have no charge, and electrons have a negative charge. These particles are arranged in specific energy levels around the nucleus of the atom.
The nucleus of an atom is the central part and contains the protons and neutrons. Protons determine the identity of an element, as the number of protons is unique to each element. Neutrons are responsible for adding stability to the nucleus. Electrons, on the other hand, orbit around the nucleus in energy levels, or shells. The number of electrons in an atom is equal to the number of protons, ensuring that the atom is electrically neutral.
The structure of an atom can be visualized as a mini solar system, with the nucleus as the sun and the electrons as the planets. However, it is important to note that the actual arrangement of electrons is not as simplistic as this analogy suggests. Electrons occupy specific regions of space known as atomic orbitals, which represent the probability of finding an electron in a particular location.
Atoms can combine with other atoms to form molecules through chemical bonding. When atoms come together, their valence electrons interact, leading to the formation of various compounds. The physical and chemical properties of elements are determined by the number and arrangement of their atoms.
In summary, atoms are the building blocks of matter and consist of protons, neutrons, and electrons arranged in energy levels around a nucleus. The structure of an atom is complex, with electrons occupying atomic orbitals. Atoms can combine with other atoms to form molecules and ultimately determine the properties of elements.
The Discovery of the Electron
The discovery of the electron was a groundbreaking scientific achievement that revolutionized our understanding of the structure of the atom. It was through the work of J.J. Thomson and his experiments with cathode rays that the existence of the electron was first proposed.
Thomson’s experiments involved the use of a cathode ray tube, a sealed glass tube with a cathode (negative electrode) and an anode (positive electrode) at opposite ends. When a high voltage was applied to the electrodes, a mysterious ray was emitted from the cathode and traveled towards the anode. This ray was later identified as a stream of negatively charged particles – the electron.
To further investigate the properties of these particles, Thomson conducted experiments with magnetic and electric fields. He discovered that the path of the cathode rays could be manipulated by these fields, indicating that they were charged particles. Additionally, he found that the ratio of the charge to the mass of the electron was always the same, regardless of the material used to produce the cathode rays.
This led Thomson to propose his famous “plum pudding” model of the atom, where he suggested that the atom was made up of a positively charged “pudding” with negatively charged electrons embedded within it. This model provided a new understanding of atomic structure and laid the foundation for future discoveries in the field of nuclear physics.
In conclusion, the discovery of the electron was a pivotal moment in the history of science. It not only confirmed the existence of subatomic particles but also paved the way for further investigations into the nature of matter. Thomson’s experiments and subsequent model of the atom revolutionized our understanding of the atomic structure and set the stage for the development of modern atomic theory.
Rutherford’s Gold Foil Experiment
The gold foil experiment, conducted by Ernest Rutherford in 1911, played a crucial role in shaping our understanding of the structure of the atom. This experiment was designed to test the prevailing model of the atom at the time, known as the “plum pudding” model, proposed by J.J. Thomson. According to this model, the atom was believed to be a uniform sphere of positive charge with negatively charged electrons scattered throughout, like plums in a pudding.
To test this model, Rutherford and his team aimed a beam of alpha particles, which are positively charged particles, at a thin sheet of gold foil. They expected that the alpha particles would pass through the foil with only slight deflections, as predicted by the plum pudding model. However, to their surprise, some of the alpha particles were deflected at large angles and even bounced back in the direction opposite to their original path.
This unexpected result led Rutherford to propose a new model of the atom. He suggested that the atom must have a tiny, dense, and positively charged nucleus at its center, which is responsible for the large deflections observed. The rest of the atom, he proposed, is mostly empty space with electrons orbiting around the nucleus. This new model, known as the “planetary model” or the “nuclear model,” laid the foundation for our modern understanding of the atom.
The gold foil experiment provided strong evidence for the existence of the nucleus and challenged the plum pudding model. It demonstrated that the atom is not a uniform sphere, but rather consists of a small, dense, and positively charged nucleus surrounded by negatively charged electrons. This experiment revolutionized the field of atomic physics and paved the way for further discoveries, such as the understanding of atomic structure, the development of quantum mechanics, and the discovery of subatomic particles.
The Bohr Model of the Atom
The Bohr Model of the Atom, also known as the Rutherford-Bohr Model, is a representation of the atom that was proposed by Danish physicist Niels Bohr in 1913. This model revolutionized our understanding of atomic structure and laid the foundation for modern quantum theory.
According to the Bohr Model, the atom consists of a nucleus at the center, which contains positively charged protons and uncharged neutrons. Electrons, which are negatively charged, orbit around the nucleus in specific energy levels or shells. These energy levels are quantized, meaning that electrons can only occupy certain discrete energy states. Furthermore, only a certain number of electrons can be accommodated within each energy level, with the innermost shell having a lower maximum capacity compared to the outer ones.
The Bohr Model also introduced the concept of electron transitions. Electrons can absorb energy and move to higher energy levels, or they can release energy and move to lower energy levels. When an electron moves to a lower energy level, it emits a specific amount of energy in the form of electromagnetic radiation, which can be observed as light. This is the basis for the study of atomic spectra, where each element produces a unique pattern of emitted light, allowing scientists to identify elements based on their spectral lines.
The Bohr Model marked a significant step forward in our understanding of atomic structure, as it provided a mathematical framework to explain the stability and behavior of the atom. However, it was later superseded by more advanced models, such as the quantum mechanical model, which provided a more accurate and comprehensive description of atomic behavior.
The Quantum Mechanical Model of the Atom
The development of the quantum mechanical model revolutionized our understanding of the atom. This model, also known as the electron cloud model or the wave mechanical model, describes the behavior of electrons in atoms. Unlike the earlier Bohr model, which depicted electrons as orbiting the nucleus in defined paths, the quantum mechanical model views electrons as existing in atomic orbitals, which are regions of space where the probability of finding an electron is the highest. These orbitals are often represented as three-dimensional shapes.
The quantum mechanical model is based on the principles of quantum mechanics, a branch of physics that deals with the behavior of particles at the atomic and subatomic level. According to quantum mechanics, electrons can be described as both particles and waves. This means that they have both particle-like properties, such as mass and charge, and wave-like properties, such as wavelength and frequency.
The quantum mechanical model also introduces the concept of electron spin. Each electron in an atom can have one of two possible spin states: spin up or spin down. This property is important in determining the arrangement of electrons in the atom and their interactions with each other.
In summary, the quantum mechanical model provides a more accurate and comprehensive understanding of the atom compared to earlier models. It incorporates the dual nature of electrons and introduces the concept of atomic orbitals, which describe the probability distribution of electrons in an atom. This model has greatly contributed to our understanding of chemical bonding, the properties of elements, and the behavior of matter at the atomic level.
Electrons in Energy Levels
Electrons are subatomic particles that have a negative charge. They are located outside of the nucleus of an atom and are organized into energy levels. Energy levels are regions of space around the nucleus where electrons are most likely to be found. These energy levels are also known as electron shells or orbitals.
Electrons in an atom occupy the lowest energy levels first before moving to higher energy levels. The energy levels are numbered with the lowest energy level being closest to the nucleus. Each energy level can hold a specific maximum number of electrons. The first energy level can hold a maximum of 2 electrons, the second energy level can hold a maximum of 8 electrons, the third energy level can hold a maximum of 18 electrons, and so on.
Electrons within an energy level are arranged in sublevels or subshells. Each sublevel has a specific shape and can hold a maximum number of electrons. The sublevels are labeled as s, p, d, and f. The s sublevel can hold a maximum of 2 electrons, the p sublevel can hold a maximum of 6 electrons, the d sublevel can hold a maximum of 10 electrons, and the f sublevel can hold a maximum of 14 electrons.
The arrangement of electrons within energy levels and sublevels follows a specific pattern called the Aufbau principle. According to this principle, electrons fill the lowest energy levels first before moving to higher energy levels, and within each energy level, electrons fill the sublevels in a specific order. This order is based on the energy of the sublevels and is known as the Aufbau sequence.
In summary, electrons in an atom are organized into energy levels or electron shells. Each energy level can hold a maximum number of electrons, and within each energy level, electrons fill sublevels in a specific order. The arrangement of electrons follows the Aufbau principle, filling the lowest energy levels first before moving to higher energy levels.
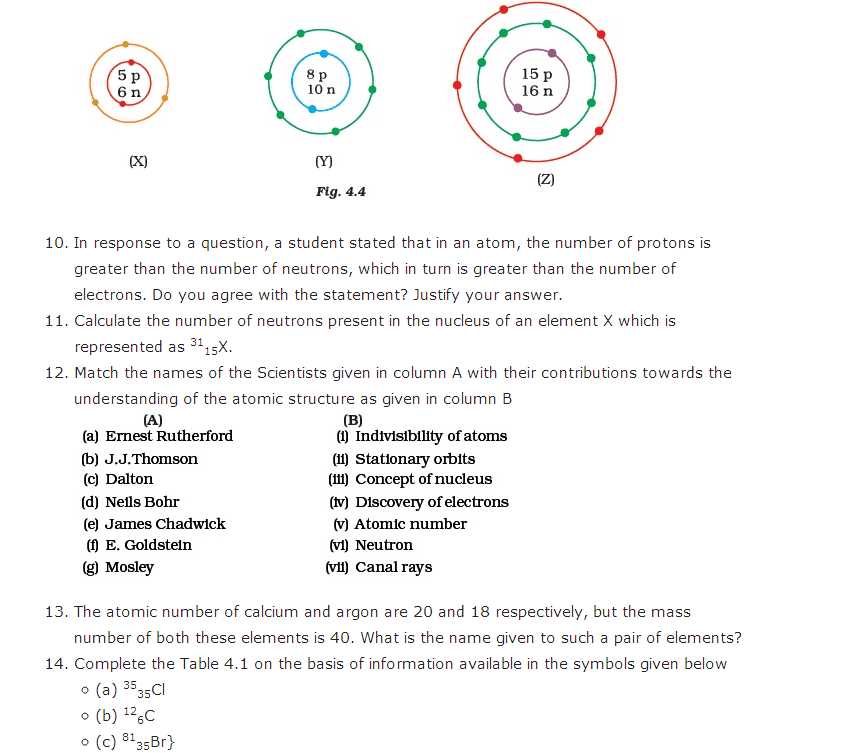
Atomic Number and Mass Number
The atomic number and the mass number are two important properties of an atom. The atomic number, denoted by the symbol Z, represents the number of protons in the nucleus of an atom. It is a unique identifier for each element on the periodic table. For example, the atomic number of hydrogen is 1, carbon is 6, and oxygen is 8.
The mass number, denoted by the symbol A, represents the total number of protons and neutrons in the nucleus of an atom. It is a whole number and is specific to each isotope of an element. Isotopes are atoms of the same element that have different numbers of neutrons. For example, carbon-12 has a mass number of 12 because it has 6 protons and 6 neutrons, while carbon-14 has a mass number of 14 because it has 6 protons and 8 neutrons.
Atomic Number: The atomic number determines the identity of an element. For example, all atoms with an atomic number of 6 are carbon atoms, regardless of their mass number or number of neutrons.
Note: The atomic number is equal to the number of electrons in a neutral atom. In a neutral atom, the number of protons is equal to the number of electrons, resulting in a net charge of zero.
Example:
Let’s take the example of oxygen. The atomic number of oxygen is 8, which means it has 8 protons in its nucleus. This also means that a neutral oxygen atom has 8 electrons orbiting the nucleus.
Mass Number: The mass number represents the total number of protons and neutrons in an atom. It is not a fixed value and can vary for different isotopes of the same element.
Note: The mass number is not listed on the periodic table since it varies for different isotopes. Instead, the average atomic mass is listed, which takes into account the abundance of each isotope.
Example:
Let’s continue with the example of oxygen. Oxygen has three naturally occurring isotopes: oxygen-16, oxygen-17, and oxygen-18. Oxygen-16 has a mass number of 16, oxygen-17 has a mass number of 17, and oxygen-18 has a mass number of 18.
Relationship between Atomic Number and Mass Number: The atomic number and mass number provide crucial information about an atom. By knowing the atomic number, we can determine the element and its position on the periodic table. The mass number gives us insight into the number of protons and neutrons in the nucleus, allowing us to distinguish different isotopes of the same element.
Summary:
- The atomic number represents the number of protons in an atom, and it is unique to each element.
- The mass number represents the total number of protons and neutrons in an atom, and it varies for different isotopes of the same element.
- The atomic number determines the identity of an element, while the mass number provides information about the number of protons and neutrons in the nucleus.