
Chemistry is a crucial subject for anyone interested in understanding the composition, structure, properties, and changes of matter. Chapter 5 is an important section of the chemistry curriculum, focusing on key topics such as chemical reactions, stoichiometry, and balancing chemical equations.
The Chapter 5 chemistry test is designed to assess students’ knowledge and comprehension of these fundamental concepts. It serves as a crucial milestone in the learning process, allowing students to demonstrate their understanding of how molecules interact, how reactions occur, and how to predict the products of chemical reactions.
During the Chapter 5 chemistry test, students are expected to apply their knowledge of chemical reactions to different scenarios and solve problems related to stoichiometry and balancing chemical equations. They may be required to identify the reactants and products of a given reaction, calculate the amount of product produced, or balance an unbalanced chemical equation.
To succeed on the Chapter 5 chemistry test, students should review and understand concepts such as the law of conservation of mass, mole-to-mole ratios, limiting reactants, and percent yield. They should also practice applying these concepts to various problems and scenarios to build their problem-solving skills and confidence in their abilities.
Chapter 5 Chemistry Test: The Basics of Chemical Reactions
Chemical reactions are fundamental processes that occur in nature, shaping the world around us. In Chapter 5 of our chemistry course, we covered the basics of chemical reactions, delving into the mechanisms that govern these transformations. Understanding chemical reactions is crucial for numerous fields, including medicine, environmental science, and materials engineering.
A chemical reaction occurs when one or more substances, known as reactants, interact to form new substances, called products. This transformation involves the breaking and formation of chemical bonds. During the test, students were evaluated on their ability to identify the reactants and products, as well as their understanding of the balanced chemical equations that represent these reactions.
Students were introduced to various types of chemical reactions, such as synthesis reactions, decomposition reactions, and combustion reactions. They were challenged to recognize the distinguishing features of each type and identify examples from real-life scenarios. By understanding the different types of reactions and their underlying mechanisms, students gained a deeper comprehension of the fundamental principles that govern chemical transformations.
The test also assessed students’ understanding of stoichiometry, a crucial concept in chemical reactions. Stoichiometry involves the quantitative relationships between the amounts of reactants and products in a chemical equation. Students were required to perform stoichiometric calculations, balancing equations and determining the ratios of substances involved.
- Reactants and products
- Types of chemical reactions
- Synthesis, decomposition, and combustion reactions
- Stoichiometry
This chapter constituted an essential foundation for more advanced topics in chemistry. By mastering the basics of chemical reactions, students gained the tools necessary to understand and predict more complex reactions and delve deeper into the fascinating world of chemistry.
Understanding Chemical Reactions: A Key Concept in Chemistry
Chemical reactions are at the core of chemistry, serving as the foundation for understanding how different substances interact and transform. These reactions involve a rearrangement of atoms, resulting in the formation of new substances with unique properties. The ability to comprehend and predict chemical reactions is a key concept in chemistry, enabling scientists to manipulate and control matter for various applications.
Atoms and Molecules: Atoms are the building blocks of matter, and they combine to form molecules. Chemical reactions involve the breaking and forming of chemical bonds between atoms, resulting in the rearrangement of these atoms to create different molecules. Understanding the composition and behavior of atoms and molecules is crucial for comprehending chemical reactions.
Reactants and Products: Chemical reactions have two main components: reactants and products. Reactants are the starting substances that undergo a change, while products are the new substances formed after the reaction. Balancing chemical equations is a fundamental skill in chemistry, ensuring that there is an equal number of atoms on both sides of the equation.
Types of Chemical Reactions: Chemical reactions can be classified into several types, including synthesis, decomposition, combustion, displacement, and double displacement reactions. Each type has its own set of characteristics and can be identified by the specific changes that occur during the reaction. Understanding these different types of reactions is essential for predicting and analyzing chemical processes.
Reaction Rates: The rate at which a chemical reaction occurs is an important factor in understanding and controlling chemical reactions. Factors such as temperature, concentration, catalysts, and surface area can influence the rate of a reaction. Studying reaction rates allows scientists to optimize conditions for desired reactions and develop efficient processes in various industries.
Applications: Understanding chemical reactions has numerous real-world applications. From the production of medications and polymers to the development of renewable energy sources and environmental remediation, knowledge of chemical reactions is crucial in designing and improving processes that impact our daily lives. Additionally, understanding reactions at the molecular level aids in the development of new materials with enhanced properties.
In conclusion, understanding chemical reactions is a key concept in chemistry. It provides the foundation for comprehending how substances interact and transform, and allows scientists to manipulate matter for various applications. By studying atoms and molecules, balancing chemical equations, identifying different types of reactions, investigating reaction rates, and applying this knowledge in various industries, scientists can make significant advancements in fields ranging from healthcare to energy.
The Importance of Chapter 5: Building a Foundation for Advanced Chemistry
In the study of chemistry, Chapter 5 plays a crucial role in building a solid foundation that will support further exploration and understanding of advanced concepts. This chapter focuses on fundamental principles and concepts that are essential for comprehending more complex topics in chemistry. By grasping these foundational ideas, students will be equipped with the necessary tools and knowledge to excel in advanced chemistry.
One key aspect of Chapter 5 is the introduction to atomic structure and the periodic table. Understanding the structure of atoms and how they are organized in the periodic table lays the groundwork for comprehending various chemical properties and behaviors. From the arrangement of electrons in an atom to the identification of elements and their characteristics, this knowledge is crucial for analyzing chemical reactions and predicting their outcomes. It provides a basis for understanding how different elements interact and contribute to the formation of compounds.
Another important topic covered in Chapter 5 is chemical bonding and molecular structure. Understanding how atoms combine to form molecules and the different types of chemical bonds that hold these molecules together is essential for comprehending and predicting chemical reactions. It provides insight into the properties and behavior of substances, allowing scientists to design and synthesize new materials with desired functionalities. By studying chemical bonding, students develop an understanding of the different forces at play in chemical reactions and gain the ability to manipulate these forces to create desired outcomes.
In addition to these foundational concepts, Chapter 5 also covers other important topics such as the properties of gases, solutions, and acids and bases. Each of these areas builds upon the foundational knowledge gained in earlier chapters, further expanding students’ understanding of the principles that govern chemical behavior.
In conclusion, Chapter 5 serves as a crucial building block for advanced chemistry by introducing foundational concepts and principles that are necessary for understanding more complex topics. By mastering these fundamentals, students will be equipped with the necessary tools to analyze and predict chemical reactions, comprehend the behavior of substances, and apply their knowledge to real-world scenarios. Chapter 5 sets the stage for further exploration and lays the groundwork for continued success in the study of chemistry.
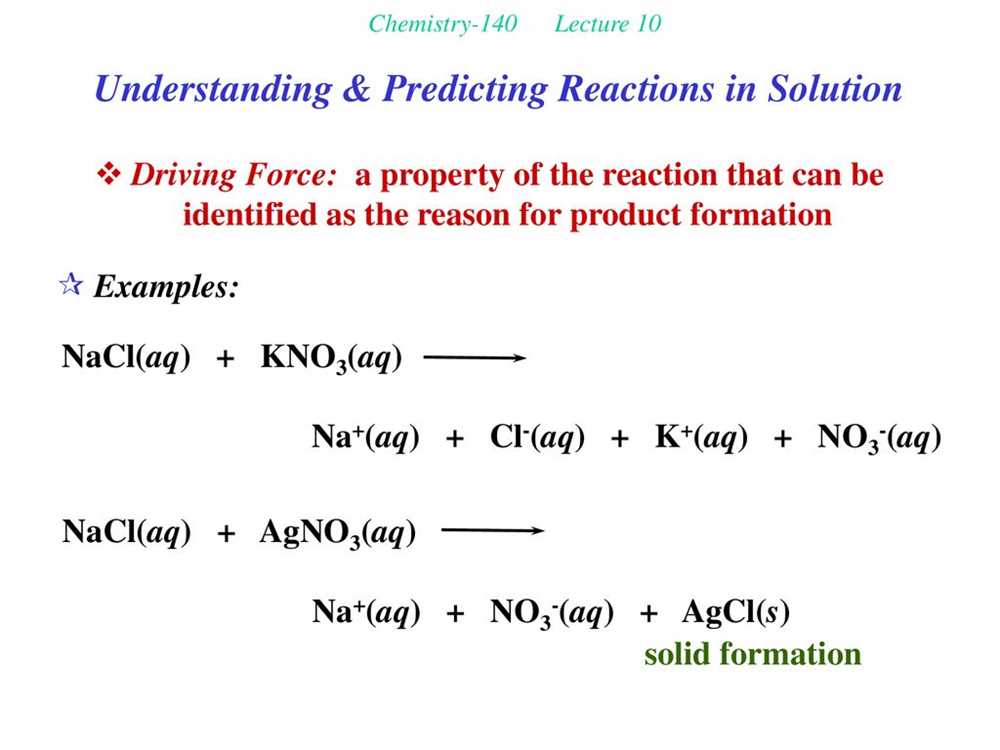
Overview of Chemical Equations: Balancing and Interpreting Reactions
Chemical equations are a representation of chemical reactions, which show the reactants and products involved in a reaction. These equations are written using chemical formulas and symbols to indicate the substances involved. Balancing a chemical equation is necessary to ensure that the law of conservation of mass is upheld, meaning that the number of atoms of each element is the same on both sides of the equation.
When balancing a chemical equation, coefficients are added in front of the formulas to indicate the number of each molecule or atom present. This is done by adjusting the coefficients until the number of atoms of each element is equal on both sides. It is important to note that subscripts cannot be changed in a balanced equation, as these represent the ratio of atoms within a molecule. Balancing equations requires a good understanding of chemical formulas and the ability to apply mathematical concepts.
Interpreting chemical reactions
Chemical equations provide valuable information about the nature of chemical reactions. They show the reactants that are consumed during the reaction and the products that are formed. The reactants are written on the left side of the equation, while the products are written on the right side. The arrow in the middle of the equation indicates the direction of the reaction.
Chemical equations can also provide information about the stoichiometry of a reaction, which refers to the ratio of reactants and products in a balanced equation. This allows chemists to calculate the amount of a substance that reacts or is produced in a given reaction. The coefficients in the balanced equation can be used as conversion factors in stoichiometric calculations.
Overall, chemical equations play a crucial role in understanding and predicting chemical reactions. Balancing equations ensures that the law of conservation of mass is upheld, while interpreting equations provides valuable information about the reactants, products, and stoichiometry of the reaction.
Types of Chemical Reactions: Exploring Acid-Base, Redox, and More
Chemical reactions are fundamental processes that occur in nature and play a crucial role in our daily lives. Understanding the types of chemical reactions is essential for predicting and explaining the behavior of substances. In this article, we will explore some of the most common types of chemical reactions, including acid-base reactions, redox reactions, and more.
Acid-Base Reactions
Acid-base reactions occur when an acid and a base react to form a salt and water. These reactions involve the transfer of protons (H+) from the acid to the base. The strength of the acid and base determines the extent of the reaction. For example, in a strong acid-strong base reaction, complete ionization occurs, resulting in the formation of water and a salt. In contrast, in a weak acid-weak base reaction, only partial ionization occurs, leading to the formation of an equilibrium mixture of the acid, base, salt, and water.
Redox Reactions
Redox reactions, also known as oxidation-reduction reactions, involve the transfer of electrons between reactants. In a redox reaction, one substance is oxidized (loses electrons) while another is reduced (gains electrons). The oxidation state of the elements in the reactants changes as a result of the electron transfer. Redox reactions are crucial in various processes, such as combustion, corrosion, and cellular respiration.
Combination Reactions

Combination reactions, also called synthesis reactions, involve the combination of two or more substances to form a single product. These reactions typically occur between elements or compounds and result in the formation of a new compound. For example, the combination of hydrogen gas (H2) and oxygen gas (O2) produces water (H2O) in a synthesis reaction.
Decomposition Reactions
Decomposition reactions involve the breakdown of a compound into its constituent elements or simpler compounds. This type of reaction is the opposite of a combination reaction. Heat, light, or the presence of a catalyst can facilitate decomposition reactions. An example of a decomposition reaction is the breakdown of hydrogen peroxide (H2O2) into water (H2O) and oxygen gas (O2).
Single Replacement Reactions
In single replacement reactions, an element replaces another element in a compound, resulting in the formation of a new compound and a different element. These reactions often occur between a metal and a salt solution or between a nonmetal and an acid. The reactivity of elements determines whether a single replacement reaction can occur. An example is the reaction between zinc (Zn) and hydrochloric acid (HCl) to produce zinc chloride (ZnCl2) and hydrogen gas (H2).
These are just a few examples of the types of chemical reactions that occur in the world around us. By understanding the principles behind these reactions, scientists can harness their power to develop new materials, improve industrial processes, and advance our understanding of the natural world.
Stoichiometry: Calculating Reactants and Products
Stoichiometry is a critical concept in chemistry that involves calculating the quantities of reactants and products in a chemical reaction. It is essential for determining the amount of each substance needed for a reaction to occur and the amount of each substance produced as a result of the reaction.
One of the key aspects of stoichiometry is the use of balanced chemical equations. These equations provide the necessary information about the ratios of reactants and products involved in a reaction. By knowing the balanced equation, chemists can determine the amount of one substance based on the amount of another.
To calculate the quantities of reactants and products, stoichiometric calculations are employed. These calculations involve using mole ratios derived from the balanced chemical equation. The mole ratio represents the ratio of moles of one substance to moles of another substance in the reaction. By multiplying the given quantity of one substance by the appropriate mole ratio, the quantity of another substance can be determined.
Stoichiometry calculations can be used in various applications, such as determining the amount of reactants needed in a reaction to produce a desired amount of product. Additionally, these calculations are vital in determining the theoretical yield of a reaction, which represents the maximum amount of product that can be obtained based on the quantities of reactants.
In summary, stoichiometry plays a crucial role in calculating the amounts of reactants and products in a chemical reaction. By using balanced chemical equations and mole ratios, chemists can accurately determine the quantities of substances involved in a reaction and predict the maximum amount of product that can be obtained.