
Chemistry is a complex and fascinating subject that seeks to understand the properties, composition, and behavior of matter. In Chapter 7 of our chemistry textbook, we explored various topics related to chemical reactions and equations. This chapter is crucial for building a solid foundation in chemistry as it introduces students to fundamental concepts such as balancing chemical equations, stoichiometry, and reaction types.
Throughout the chapter, students were presented with several challenging questions and problems to test their understanding of these concepts. In this article, we will provide answers to some of the key questions from the Chapter 7 test, offering a comprehensive review of the material covered.
We will begin by discussing how to balance chemical equations, a fundamental skill necessary for understanding and predicting the outcome of chemical reactions. Balancing chemical equations involves ensuring that the number of atoms of each element is the same on both sides of the equation. This is achieved by adjusting the coefficients in front of each compound or element in the equation. We will provide step-by-step solutions to example problems, illustrating the process of balancing equations.
Chapter 7 Chemistry Test Answers
Here are the answers to the Chapter 7 Chemistry test:
- What is the atomic number? The atomic number is the number of protons in an atom’s nucleus.
- How do you calculate the atomic mass? The atomic mass is calculated by summing the masses of the atom’s protons and neutrons.
- What is the difference between an element and a compound? An element is a substance that cannot be broken down into simpler substances, while a compound is made up of two or more different elements chemically combined.
- What is an isotope? An isotope is an atom that has the same number of protons but a different number of neutrons. This results in a different atomic mass.
- Define a mole. A mole is a unit of measurement in chemistry that represents the amount of a substance. One mole is equal to Avogadro’s number, which is approximately 6.022 x 10^23.
- What is the Law of Conservation of Mass? The Law of Conservation of Mass states that mass cannot be created or destroyed in a chemical reaction. The total mass of the reactants is equal to the total mass of the products.
- Explain the concept of molar mass. Molar mass is the mass of one mole of a substance. It is calculated by summing the atomic masses of all the atoms in a molecule.
- What is the difference between an empirical formula and a molecular formula? An empirical formula gives the simplest whole number ratio of atoms in a compound, while a molecular formula gives the actual number of atoms of each element in a molecule.
- What is stoichiometry? Stoichiometry is the calculation of quantities in a chemical reaction. It involves using balanced chemical equations to determine the mole ratios between reactants and products.
These are just a few of the answers to the Chapter 7 Chemistry test. It is important to study and understand all the concepts covered in the chapter to be successful on the test and in further studies in chemistry.
Overview of Chapter 7 Chemistry Test
In Chapter 7 of your chemistry textbook, you have learned about several important topics related to chemical reactions. This chapter explores the concept of chemical equilibrium, which is the state in which the rates of the forward and reverse reactions are equal. You have also studied the factors that affect chemical equilibrium, such as concentration, temperature, and pressure. Understanding these factors is crucial in predicting and manipulating chemical reactions.
Another key topic covered in Chapter 7 is the Le Chatelier’s principle, which states that when a system at equilibrium is subjected to a stress, it will respond in such a way to counteract the stress and restore equilibrium. This principle helps us understand how changes in concentration, temperature, or pressure can shift the equilibrium position of a reaction. It is important to remember the effect of these changes on the equilibrium constant and the direction in which the reaction will shift.
- In the upcoming Chapter 7 chemistry test, you can expect questions that require you to apply the concepts of chemical equilibrium, Le Chatelier’s principle, and the factors affecting equilibrium.
- You will be tested on your understanding of how changes in concentration, temperature, and pressure can impact the equilibrium position of a reaction.
- Be prepared to solve equilibrium problems, such as calculating equilibrium constants and predicting the shift in equilibrium based on changes in conditions.
It is recommended to review your class notes, the chapter summary, and practice problems to ensure that you are well-prepared for the Chapter 7 chemistry test. Understanding the key concepts and being able to apply them in various scenarios will help you excel in the test. Good luck!
Answering Multiple Choice Questions
Answering multiple choice questions can be challenging, as these questions require you to select the correct answer from several options provided. However, with the right approach, you can improve your chances of answering correctly and achieving a higher score on your chemistry test. Here are some strategies to keep in mind:
1. Read the question carefully: Take your time to understand what the question is asking. Pay attention to keywords, such as “not,” “except,” or “best,” as they can completely change the meaning of the question.
2. Eliminate incorrect options: Go through the options and eliminate any that you know are incorrect. This can help narrow down your choices and increase your chances of selecting the correct answer.
- 3. Use the process of elimination: If you’re unsure about the correct answer, try to eliminate more options by using your knowledge of the subject. Cross out any options that you know are incorrect and focus on the remaining choices.
- 4. Trust your instincts: Sometimes your initial gut reaction is the correct answer. Trust your instincts and go with the option that feels most right to you.
- 5. Use context clues: If a question refers to a specific concept or topic in the chapter, use your knowledge of that topic to help you choose the correct answer. Look for clues in the question itself or in the surrounding questions to guide your decision.
- 6. Avoid overthinking: Sometimes the correct answer is more straightforward than you think. Avoid overanalyzing the options and stick to what you know.
Remember, practice makes perfect. The more you familiarize yourself with multiple choice questions and the content of the chapter, the better prepared you will be to answer them accurately. Don’t forget to review your answers and learn from any mistakes you make. Good luck!
Answering Fill-in-the-Blank Questions
Fill-in-the-blank questions are a common type of assessment used in chemistry exams. These questions require students to recall specific information and provide the missing word or phrase to complete a sentence or statement. Answering fill-in-the-blank questions correctly requires a solid understanding of the subject matter and the ability to recall relevant information accurately.
When answering fill-in-the-blank questions, it is important to carefully read the entire sentence or statement to understand the context and determine the missing word or phrase. Pay attention to any clues or keywords that may help you identify the correct answer. Some questions may provide a word bank or list of options to choose from, while others may require you to generate the answer independently.
Tips for answering fill-in-the-blank questions:
- Review the material: Before the test, make sure you have a thorough understanding of the concepts and information covered in the chapter. Review your notes, textbook, and any other relevant resources.
- Predict the answer: After reading the question, try to predict the missing word or phrase based on your knowledge of the topic. This can help guide your thinking and increase the chances of selecting the correct answer.
- Eliminate options: If the question provides a list of options, carefully evaluate each one. Eliminate any options that are clearly incorrect or do not fit the context of the sentence. This can help narrow down the possibilities and improve your chances of selecting the correct answer.
- Check for grammar and context: Ensure that the answer you provide grammatically and contextually makes sense in the given sentence or statement. Double-check for any errors or inconsistencies before finalizing your answer.
- Be cautious with similar options: Some fill-in-the-blank questions may have options that are very similar in meaning or spelling. Take extra care to differentiate between these options and select the most appropriate one.
By following these tips and practicing answering fill-in-the-blank questions, you can improve your skills in recalling information accurately and successfully completing this type of assessment in your chemistry exams.
Answering True or False Questions
Answering true or false questions is a common task in exams and tests, especially in subjects like chemistry. These types of questions require a careful evaluation of the statement provided and determining its accuracy. It is important to approach these questions with a clear understanding of the subject matter and the ability to distinguish between true and false statements.
1. Understand the question: Before answering a true or false question, it is crucial to understand what is being asked. Read the statement carefully and make sure to grasp its meaning. Pay attention to any specific keywords or phrases that may alter the statement’s accuracy.
2. Analyze the information: Once you understand the question, analyze the information provided in the statement. Evaluate the statement based on your knowledge of the topic and any relevant facts or principles. Consider whether the statement aligns with the established facts or theories in the subject area.
3. Beware of absolutes: Statements that include absolutes like “always,” “never,” or “completely” often require extra caution. These absolute terms suggest that the statement must be true in all situations. If you can find a single exception or counterexample to the statement, then it is likely false.
4. Use logical reasoning: Apply logical reasoning and critical thinking skills to analyze the statement. Consider the context of the question and any supporting evidence or examples that may be relevant. Look for any inconsistencies or contradictions within the statement.
5. Eliminate extremes: Statements that present extreme or exaggerated claims should be approached with skepticism. Often, these statements can be false or misleading. Look for more balanced and reasonable statements that align with the general principles and concepts of the subject.
To summarize, answering true or false questions requires careful evaluation, understanding of the subject matter, and the ability to analyze the information provided. Follow these steps to increase your accuracy in answering such questions and ensure a higher chance of success in exams and tests.
Answering Short Answer Questions
Answering short answer questions in a test can be challenging, but with the right approach and preparation, it can also be an opportunity to showcase your knowledge and understanding of the subject matter. Here are some tips to help you effectively answer short answer questions:
1. Understand the question
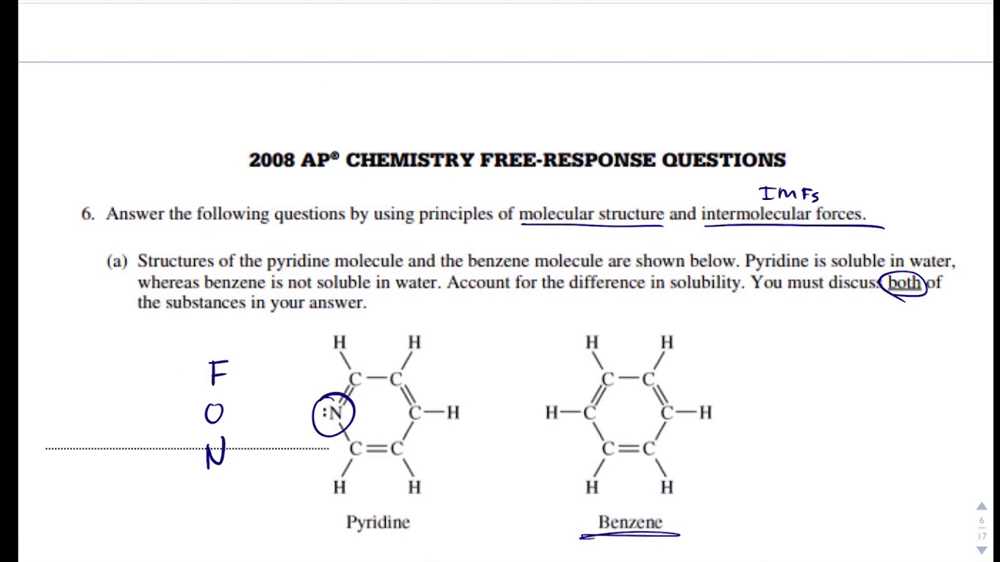
Before you begin answering a short answer question, make sure you fully understand what is being asked. Read the question carefully and identify key terms or concepts that need to be addressed in your response. Pay attention to any specific instructions, such as providing examples or comparing and contrasting certain factors.
2. Plan your response
Once you have a clear understanding of the question, take a moment to plan your response. Consider the main points or arguments you want to make and how you can structure your answer to effectively address the question. This will help you stay organized and ensure that you cover all necessary points.
3. Provide clear and concise answers
When answering short answer questions, it is important to be clear and concise in your response. Use proper grammar, punctuation, and spelling to effectively communicate your ideas. Avoid unnecessary repetition or rambling and focus on providing direct and specific answers to the question.
4. Support your answers with evidence

In order to strengthen your answers, it is crucial to support them with evidence. This can include citing relevant examples, referring to specific sources, or applying principles or theories from the subject matter. Providing evidence shows that you have a deep understanding of the topic and can help validate your arguments.
5. Review and revise
Before submitting your answers, take the time to review and revise your responses. Check for any errors, inconsistencies, or missing information. Ensure that your answers are logical, well-structured, and effectively address the question at hand. Making these final adjustments can greatly enhance the quality of your answers.
By following these guidelines, you can approach short answer questions with confidence and demonstrate your knowledge and understanding effectively. Remember to stay focused, provide clear and concise answers, and support your arguments with evidence. Good luck!
Answering Calculation Questions
When it comes to answering calculation questions in chemistry, it is important to approach them systematically and accurately. These questions typically involve performing mathematical operations to determine quantities such as mass, volume, concentration, or molar ratios.
Step 1: Understand the Question
Before diving into the calculations, it is crucial to fully understand the question and what it is asking for. Take the time to read through the question carefully and identify the given information, the desired quantity, and any other relevant details.
Step 2: Set up the Calculation
Once you have a clear understanding of the question, you can begin setting up the calculation. This often involves utilizing formulas or equations that relate the given information to the desired quantity. Write down the given values, the formula, and the unknown quantity.
Step 3: Perform the Calculation
With the setup in place, it is time to perform the actual calculation. Make sure to use the correct units and pay attention to significant figures. Follow the necessary mathematical operations, such as addition, subtraction, multiplication, or division, to arrive at the final answer.
Step 4: Evaluate and Check
After obtaining the answer, it is important to evaluate if it makes sense in the context of the question. Check if the answer has the correct units and if it falls within the expected range. Additionally, double-check your calculations to ensure accuracy. Carelessness in calculation can lead to incorrect answers.
Step 5: Communicate the Answer
Finally, clearly communicate your answer. Write it down with the appropriate units and significant figures. Additionally, provide any necessary explanations or justifications for your methodology or assumptions made during the calculation process.
By following these steps and approaching calculation questions systematically, you can improve your problem-solving skills in chemistry and achieve accurate results.