
In chemistry, covalent bonding is a type of chemical bonding that involves the sharing of electron pairs between atoms. This type of bond is typically formed between nonmetallic atoms, and it plays a crucial role in the formation of molecules and compounds. Understanding covalent bonding is essential for understanding the properties and behavior of these substances.
This article will provide answers to the Chapter 8 Covalent Bonding Worksheet, which focuses on concepts such as Lewis structures, molecular geometry, and the bond properties of covalent compounds. By exploring these answers, you will gain a deeper understanding of the principles behind covalent bonding and how it influences the properties of different substances.
One of the key aspects of covalent bonding is the ability to predict the shapes of molecules based on their Lewis structures. This worksheet will challenge you to determine the molecular geometries of various covalent compounds and understand how the arrangement of atoms and electron pairs affects the overall shape of the molecule. Through these exercises, you will develop important skills in visualizing molecular structures and predicting their properties.
Chapter 8 Covalent Bonding Worksheet Answers
In Chapter 8 of the covalent bonding unit, students were given a worksheet to practice their understanding of covalent bonding and determine the answers to a series of questions. The concept of covalent bonding involves the sharing of electrons between atoms to form a stable molecule.
One of the questions on the worksheet asked students to identify the number of valence electrons in certain elements. Valence electrons are the outermost electrons in an atom, and they play a crucial role in chemical bonding. To determine the number of valence electrons, students need to refer to the periodic table and look at the group number of the element. For example, elements in Group 1A have one valence electron, while elements in Group 6A have six valence electrons.
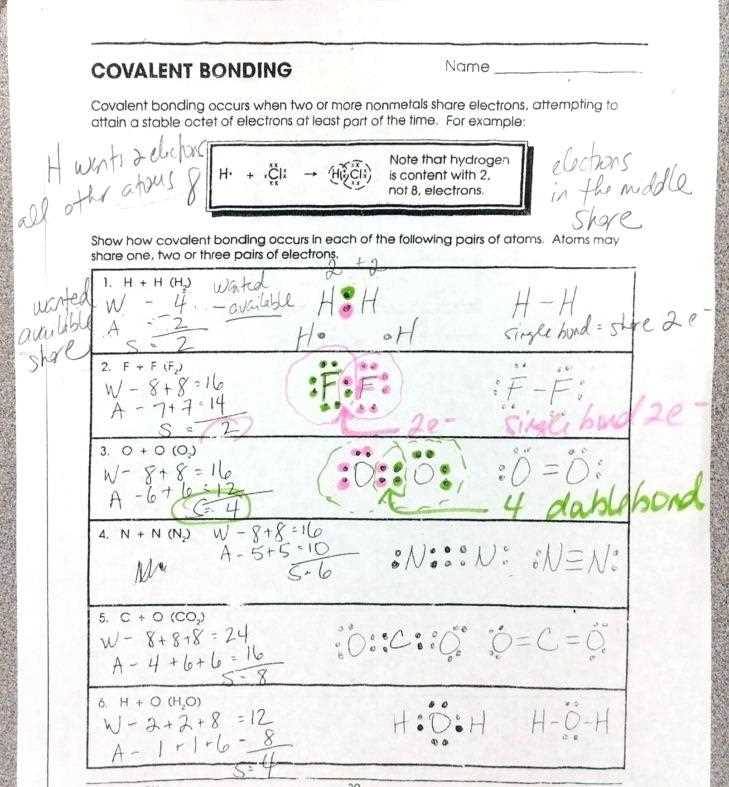
Another question on the worksheet asked students to draw Lewis dot structures for various compounds. Lewis dot structures are diagrams that represent the arrangement of valence electrons around an atom. Students need to first determine the total number of valence electrons in the compound by adding up the valence electrons of each atom. Then, they can distribute the electrons around the atoms in pairs to fulfill the octet rule, which states that atoms tend to gain, lose, or share electrons in order to achieve a full outer electron shell of eight electrons.
In addition to these questions, the worksheet also covered topics such as bond polarity, molecular geometry, and intermolecular forces. These concepts are important in understanding the physical and chemical properties of covalent compounds. By completing the worksheet and checking their answers, students can reinforce their knowledge and improve their understanding of covalent bonding.
What is covalent bonding?
In chemistry, covalent bonding is a type of chemical bonding that occurs when two or more atoms share electrons in order to achieve a stable electron configuration. This type of bonding is commonly found between nonmetallic elements, such as carbon, nitrogen, and oxygen.
Covalent bonds are formed through the overlapping of atomic orbitals, resulting in the formation of a shared electron pair. This shared electron pair is known as a bonding pair. The atoms involved in the covalent bond are held together by the attraction between the positive nuclei and the shared electrons.
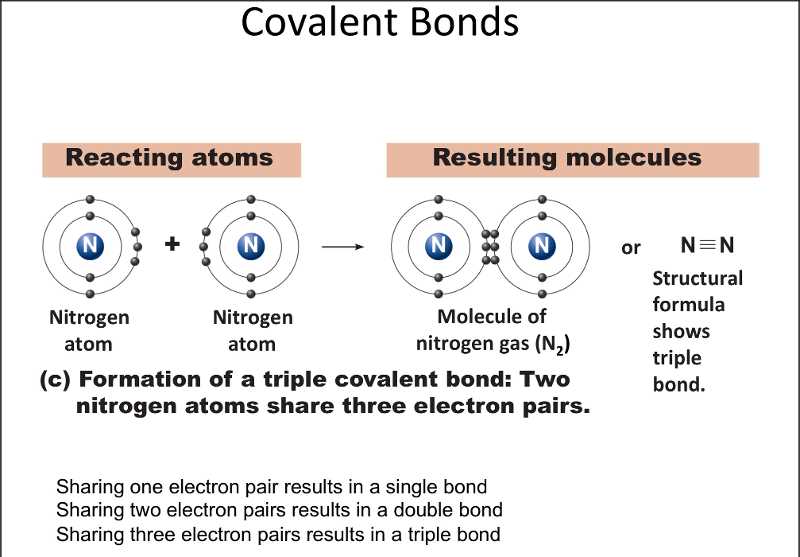
In a covalent bond, the atoms involved usually contribute one or more electrons to the shared pair. This allows each atom to achieve a full outer electron shell, which is the most stable electron configuration. The number of electrons shared between atoms can vary, leading to different types of covalent bonds, such as single, double, or triple bonds.
Covalent bonding is characterized by its strong bond strength and high melting and boiling points. It is also important in determining the physical and chemical properties of different substances. Covalent compounds can exist as gases, liquids, or solids, depending on the strength of the covalent bonds between the atoms.
In summary, covalent bonding involves the sharing of electrons between atoms, resulting in the formation of stable electron configurations. This type of bonding is common in nonmetallic elements and plays a crucial role in the formation of various compounds with unique properties.
Understanding the bonding in covalent compounds
In the world of chemistry, there are two main types of chemical bonds: ionic and covalent. While ionic bonds involve the transfer of electrons between atoms, covalent bonds occur when atoms share electrons with each other. Covalent compounds are formed through this sharing of electrons, resulting in a strong bond between the atoms.
Covalent compounds are typically formed between nonmetal elements, as these elements have similar electronegativities and can easily share electrons. The sharing of electrons allows each atom to achieve a stable electron configuration, similar to that of a noble gas. This stability is crucial for the formation of a strong covalent bond.
Understanding the bonding in covalent compounds involves analyzing the Lewis structure of the compound. In a Lewis structure, the valence electrons of each atom are represented by dots or lines, indicating the sharing of electrons. The number of shared electrons determines the type of covalent bond: single bonds involve the sharing of two electrons, double bonds involve the sharing of four electrons, and triple bonds involve the sharing of six electrons.
Covalent compounds can have different structures, such as linear, trigonal planar, tetrahedral, or even more complex arrangements. The shape of the molecule is determined by the arrangement of the atoms and the presence of lone pairs of electrons. This shape influences many properties of the compound, including its polarity and reactivity.
Overall, understanding the bonding in covalent compounds is essential for understanding the properties and behaviors of numerous substances, including organic molecules, which are the building blocks of life. By studying the structure and bonding in covalent compounds, chemists can gain valuable insights into the nature of matter and create new materials with specific properties.
Types of Covalent Bonds
In covalent bonding, atoms share electrons to form a stable chemical bond. There are three types of covalent bonds: nonpolar covalent bonds, polar covalent bonds, and coordinate covalent bonds.
Nonpolar covalent bonds occur between atoms with similar electronegativities. In these bonds, electrons are shared equally between the atoms, resulting in a symmetrical distribution of charge. This means that neither atom has a stronger pull on the shared electrons, and the bond is considered nonpolar. Examples of compounds with nonpolar covalent bonds include diatomic molecules like oxygen (O2) and nitrogen (N2).
Polar covalent bonds occur between atoms with different electronegativities. In these bonds, electrons are not shared equally, resulting in a partial positive charge on one atom and a partial negative charge on the other. The atom with the higher electronegativity has a stronger pull on the shared electrons, creating an uneven distribution of charge. Examples of compounds with polar covalent bonds include water (H2O) and hydrogen chloride (HCl).
Coordinate covalent bonds, also known as dative covalent bonds, occur when one atom donates a lone pair of electrons to another atom that needs electrons to complete its octet. This type of bond is formed when one atom acts as the donor and the other as the acceptor of electrons. Coordinate covalent bonds can be found in molecules such as ammonia (NH3) and the ammonium ion (NH4+).
- Nonpolar covalent bonds: electrons shared equally between atoms of similar electronegativity
- Polar covalent bonds: electrons shared unequally between atoms of different electronegativity, resulting in partial charges
- Coordinate covalent bonds: one atom donates a lone pair of electrons to another atom in order to complete its octet
Understanding the different types of covalent bonds is essential to fully comprehend the properties and behaviors of covalent compounds. These bonds play a fundamental role in the formation and stability of molecules, and their characteristics can impact various chemical reactions and interactions.
Naming Covalent Compounds
Covalent compounds are formed when two or more nonmetal atoms share electrons to achieve a stable electron configuration. Naming covalent compounds is based on the prefixes that indicate the number of atoms present in the compound.
When naming covalent compounds, the full name of the first nonmetal is used, followed by the prefix that corresponds to the number of its atoms. The second nonmetal’s name is then modified to end with the suffix “-ide”, and the appropriate prefix is added to indicate the number of its atoms. The prefixes used in naming covalent compounds are: mono-, di-, tri-, tetra-, penta-, hexa-, hepta-, octa-, nona-, and deca-.
For example, CO2 is named carbon dioxide because “carbon” is the full name of the first nonmetal and “dioxide” is the modified name of the second nonmetal. The prefix “di-” is used to indicate that there are two atoms of oxygen in the compound.
In cases where the prefix “mono-” is used for the first nonmetal, it is often omitted. For example, N2O is named dinitrogen monoxide because “dinitrogen” is the full name of the first nonmetal and “monoxide” is the modified name of the second nonmetal. The prefix “mono-” is used to indicate that there is only one atom of oxygen in the compound, but it is commonly left out in the naming.
Overall, naming covalent compounds involves identifying the nonmetals present in the compound, determining the number of atoms for each nonmetal, and using the appropriate prefixes to indicate the number of atoms. This naming system helps to accurately represent the composition of covalent compounds.
Lewis Structures and Molecular Geometry
Lewis structures are diagrams that show the bonding between atoms in a molecule and the arrangement of electrons in the molecule. They are named after Gilbert N. Lewis, who first introduced the concept in 1916. Lewis structures are useful tools in predicting the shapes and properties of molecules.
To draw a Lewis structure, you start by determining the total number of valence electrons in the molecule. This is done by adding up the number of valence electrons for each atom in the molecule. The valence electrons are the electrons in the outermost energy level of an atom. Once you have the total number of valence electrons, you distribute them around the atoms to satisfy the octet rule. The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a full outer shell of eight electrons.
After drawing the Lewis structure, you can determine the molecular geometry of the molecule. Molecular geometry refers to the three-dimensional arrangement of atoms in a molecule. It is determined by the number of electron pairs surrounding the central atom and the repulsion between these electron pairs. The most common molecular geometries are linear, trigonal planar, tetrahedral, trigonal bipyramidal, and octahedral.
- A linear molecular geometry has two bonded atoms and no lone pairs.
- A trigonal planar molecular geometry has three bonded atoms and no lone pairs.
- A tetrahedral molecular geometry has four bonded atoms and no lone pairs.
- A trigonal bipyramidal molecular geometry has five bonded atoms and no lone pairs.
- An octahedral molecular geometry has six bonded atoms and no lone pairs.
Knowing the molecular geometry is important because it affects the physical and chemical properties of a molecule. For example, molecules with linear geometries tend to be nonpolar, while molecules with trigonal planar or tetrahedral geometries can be polar or nonpolar depending on the presence of polar bonds and the arrangement of polar bonds in the molecule.
VSEPR theory and predicting molecular shapes
The VSEPR (Valence Shell Electron Pair Repulsion) theory is a useful tool in predicting the three-dimensional shapes of molecules. It is based on the concept that pairs of electrons in the valence shell of an atom repel each other and therefore tend to be as far apart as possible. This repulsion determines the shape of the molecule.
In VSEPR theory, the central atom in a molecule is surrounded by its bonding pairs and lone pairs of electrons. The number of bonding pairs and lone pairs around the central atom determines the molecular shape. There are different molecular geometries, including linear, trigonal planar, tetrahedral, trigonal bipyramidal, and octahedral, among others.
For example, a molecule with two bonding pairs and no lone pairs around the central atom will have a linear molecular shape. On the other hand, a molecule with four bonding pairs and no lone pairs around the central atom will have a tetrahedral shape. The VSEPR theory allows us to predict these shapes based on the number of bonding and lone pairs.
- Linear: 2 bonding pairs, 0 lone pairs
- Trigonal planar: 3 bonding pairs, 0 lone pairs
- Tetrahedral: 4 bonding pairs, 0 lone pairs
- Trigonal bipyramidal: 5 bonding pairs, 0 lone pairs
- Octahedral: 6 bonding pairs, 0 lone pairs
In addition to predicting molecular shapes, VSEPR theory also helps us understand the polarity of molecules. The geometry of a molecule determines its molecular polarity, which is important for understanding its chemical properties and interactions.
In conclusion, VSEPR theory is a valuable tool in predicting molecular shapes and understanding the three-dimensional arrangement of atoms in molecules. By considering the number of bonding and lone pairs around the central atom, we can determine the molecular geometry and gain insights into the properties and behavior of molecules.
Bond Polarity and Electronegativity
Bond polarity refers to the unequal sharing of electrons between two atoms in a chemical bond. This unequal sharing results in a positive and negative end of the bond, called a polar bond. The polarity of a bond is determined by the difference in electronegativity values between the two atoms involved in the bond.
Electronegativity is a measure of an atom’s ability to attract shared electrons towards itself in a chemical bond. It is a property of individual atoms and can be used to determine the direction and strength of the electron distribution in a bond. The higher the electronegativity value of an atom, the more it attracts the electrons in a bond towards itself.
In general, when atoms with different electronegativity values form a bond, there will be a partial positive charge on the less electronegative atom and a partial negative charge on the more electronegative atom. This creates a dipole in the bond and is referred to as a polar covalent bond.
The difference in electronegativity values can also be used to classify the type of bond formed between two atoms. If the electronegativity difference is small (less than 0.4), the bond is considered to be nonpolar covalent, meaning the electrons are shared equally between the two atoms. If the electronegativity difference is moderate (between 0.4 and 1.7), the bond is considered to be polar covalent, with unequal sharing of electrons. If the electronegativity difference is large (greater than 1.7), the bond is considered to be ionic, with a complete transfer of electrons from one atom to another.
Understanding bond polarity and electronegativity is important in predicting the physical and chemical properties of compounds, as well as understanding the nature of chemical bonds and their interactions.