Understanding the fundamental principles of gases is crucial in many scientific and engineering disciplines. One of the most important laws governing the behavior of gases is Charles’s Law, which describes the relationship between temperature and volume. By exploring a Charles Law worksheet with answers in PDF format, students and professionals alike can enhance their understanding of this fundamental concept.
Charles’s Law states that the volume of a given mass of gas is directly proportional to its temperature, assuming that the pressure and amount of gas remain constant. This law provides valuable insights into how gases behave when subjected to changes in temperature. By studying a Charles Law worksheet with answers, individuals can grasp the quantitative relationship between temperature and volume in various scenarios.
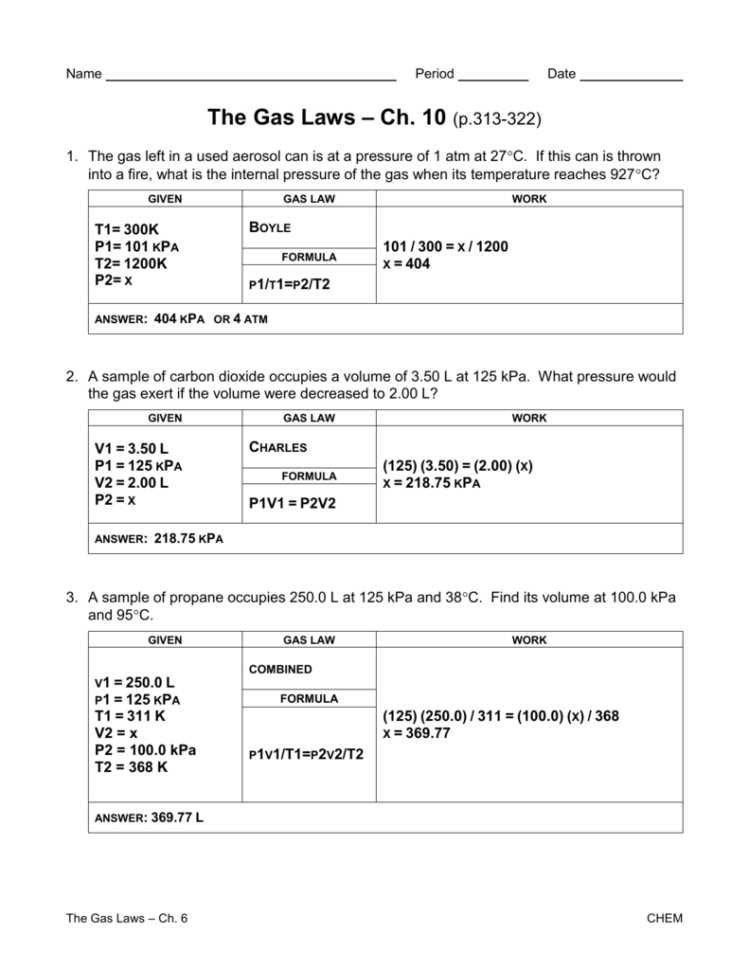
The Charles Law worksheet with answers in PDF format offers a comprehensive set of questions and solutions that enable learners to test their knowledge and practice applying the law. The worksheet covers a range of scenarios, such as calculating volume changes at different temperatures or determining temperature changes when volume is known. By working through these exercises, learners can reinforce their understanding of Charles’s Law and develop problem-solving skills.
Charles Law Worksheet with Answers PDF: What is it and why is it important?
Charles Law is a fundamental principle in the study of thermodynamics and gases. It describes the relationship between the volume and temperature of a gas at a constant pressure. Charles Law states that the volume of a gas is directly proportional to its temperature when the pressure is kept constant.
A Charles Law worksheet with answers in PDF format provides students with a practical way to apply and test their understanding of this important thermodynamic principle. These worksheets typically include a variety of questions and problems that require students to use Charles Law to calculate and predict gas behavior.
The importance of Charles Law lies in its relevance to real-world applications. Understanding this law allows scientists and engineers to make accurate predictions about how gases will behave under different temperature conditions. This is crucial in fields such as chemistry, physics, and engineering, where gases play a vital role in various processes and systems.
By using a Charles Law worksheet with answers in PDF format, students can practice solving problems and gain a deeper understanding of the relationship between temperature and volume in gases. These worksheets often include step-by-step solutions and explanations, allowing students to learn from their mistakes and improve their problem-solving skills.
Overall, Charles Law worksheets with answers in PDF format provide an effective tool for students to master this fundamental thermodynamic principle. By practicing with these worksheets, students can strengthen their knowledge and skills in applying Charles Law, which will ultimately benefit their understanding of gases and their applications in the real world.
Understanding Charles Law and its significance in thermodynamics

In the study of thermodynamics, Charles’s Law plays a crucial role in understanding the relationship between temperature and volume of a gas. It states that at constant pressure, the volume of a gas is directly proportional to its temperature. This law is named after Jacques Charles, a French scientist who first described this relationship in the late 18th century.
Charles’s Law can be mathematically represented as V1/T1 = V2/T2, where V1 and V2 are the initial and final volumes of the gas, and T1 and T2 are the initial and final temperatures, respectively. This equation shows that as the temperature of a gas increases, its volume also increases, assuming the pressure remains constant. Similarly, as the temperature decreases, the volume decreases.
This law is significant in thermodynamics for several reasons. Firstly, it helps us understand the behavior of gases when exposed to different temperatures. For example, as the temperature of a gas increases, its particles gain more kinetic energy and move faster, causing them to occupy a larger space. This knowledge is essential in a variety of industries, such as HVAC systems, where understanding the expansion and contraction of gases is crucial for proper functioning.
- Charles’s Law also provides a basis for the calculation of temperatures and volumes in various chemical reactions and processes. It allows scientists and engineers to predict the behavior of gases under different conditions and make informed decisions.
- Furthermore, this law is utilized in the development and design of various technologies, such as hot air balloons. By applying Charles’s Law, engineers can determine the relationship between the temperature of the air inside the balloon and its volume, ensuring a safe and efficient flight.
- Additionally, Charles’s Law is closely related to the ideal gas law, which combines other gas laws to describe the behavior of an ideal gas. Understanding this law is vital in the study of thermodynamics and enables scientists to accurately describe and predict the properties of gases.
In conclusion, Charles’s Law provides valuable insights into the relationship between temperature and volume of a gas. Its significance in thermodynamics lies in enabling us to understand and predict the behavior of gases under different conditions, leading to advancements in various industries and technologies. By applying this law, scientists and engineers can make informed decisions and develop innovative solutions to real-world problems.
Key Concepts covered in Charles Law Worksheet

The Charles Law Worksheet covers several key concepts related to Charles’ Law, which describes the relationship between the volume and temperature of a gas. These concepts include:
- Charles’ Law: The law states that at a constant pressure, the volume of a gas is directly proportional to its temperature in kelvin. This means that as the temperature of a gas increases, its volume also increases, and vice versa.
- Gas behavior: The worksheet explores the behavior of gases and how they respond to changes in temperature and volume. It emphasizes that gases are highly compressible and can expand or contract depending on external conditions.
- Kelvin temperature scale: The worksheet highlights the use of the kelvin temperature scale, which is based on absolute zero and is commonly used in gas laws. It explains how to convert between Celsius and kelvin temperatures.
- Direct and inverse relationships: The worksheet introduces the concept of direct and inverse relationships in science. It explains that in Charles’ Law, the relationship between volume and temperature is direct, meaning that as one variable increases, the other also increases.
- Mathematical formula: The worksheet provides the mathematical formula for Charles’ Law, which states that V1/T1 = V2/T2. It explains how to use this formula to solve problems involving volume and temperature changes.
- Sample problems: The worksheet includes sample problems that require students to apply Charles’ Law to real-world situations. These problems involve calculations and critical thinking to determine the relationships between volume and temperature in different scenarios.
By covering these key concepts, the Charles Law Worksheet helps students develop a solid understanding of the relationship between volume and temperature in gases and how to apply Charles’ Law in various situations. It provides a foundation for further study of gas laws and their practical applications.
The relationship between temperature and volume in a gas
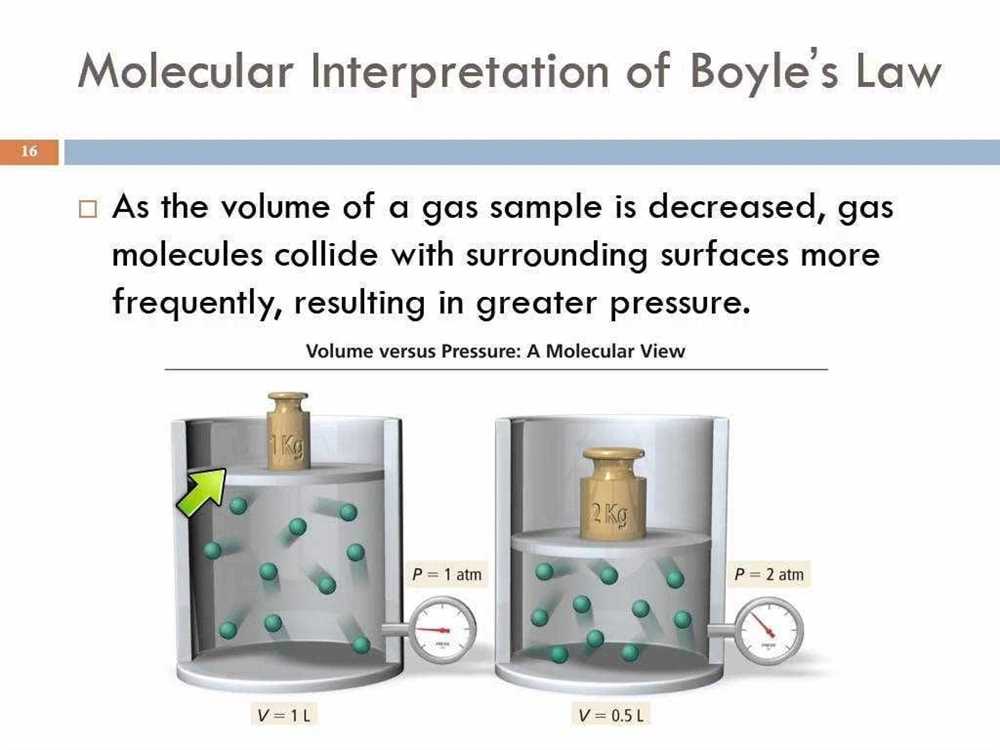
Charles’ Law states that there is a direct relationship between the temperature of a gas and its volume, assuming that the pressure remains constant. According to this law, as the temperature of a gas increases, its volume also increases proportionally, and vice versa. This relationship can be summarized in the formula V₁ / T₁ = V₂ / T₂, where V₁ and T₁ represent the initial volume and temperature, and V₂ and T₂ represent the final volume and temperature.
This principle can be explained through the kinetic theory of gases. According to this theory, gas molecules are in constant motion, and their movement is directly related to their temperature. When the temperature of a gas increases, the average kinetic energy of its molecules also increases. As a result, the gas molecules move faster and collide more frequently with the walls of the container. This increased collision frequency leads to an expansion of the gas and an increase in its volume.
Example:
Let’s consider an example to understand Charles’ Law better. Imagine a balloon filled with air. If we heat the balloon by placing it near a source of heat, such as a fire, the temperature of the air inside the balloon will increase. As a result, the molecules of the air inside the balloon will move faster and collide more frequently with the walls of the balloon. This increased collision frequency will cause the balloon to expand and increase in volume.
On the other hand, if we cool the balloon by placing it in a cold environment, the temperature of the air inside the balloon will decrease. As a result, the molecules of the air inside the balloon will move slower and collide less frequently with the walls of the balloon. This decreased collision frequency will cause the balloon to contract and decrease in volume.
In conclusion, Charles’ Law explains the relationship between temperature and volume in a gas. Understanding this relationship is essential in various scientific fields, such as chemistry, physics, and engineering, as it allows us to predict and control the behavior of gases under different temperature conditions.
Boyle’s Law and Charles Law: Similarities and Differences
Boyle’s Law and Charles Law are two fundamental principles in the field of gas laws that help us understand the behavior of gases. Although they are related to each other and describe similar concepts, there are also key differences between them. Let’s explore the similarities and differences between Boyle’s Law and Charles Law.
Similarities:
- Both Boyle’s Law and Charles Law are empirical gas laws that describe the relationship between the pressure, volume, and temperature of a gas sample.
- Both laws rely on the assumption that the amount of gas and the gas constant remain constant.
- Both laws are important in understanding and predicting the behavior of gases under different conditions.
Differences:
- Boyle’s Law states that at a constant temperature, the volume of a gas is inversely proportional to its pressure. This means that as the pressure of a gas increases, its volume decreases, and vice versa.
- Charles Law, on the other hand, states that at a constant pressure, the volume of a gas is directly proportional to its temperature. In other words, as the temperature of a gas increases, its volume also increases, and vice versa.
- Boyle’s Law only considers the relationship between pressure and volume, while Charles Law focuses on the relationship between volume and temperature.
- Boyle’s Law can be graphically represented by a hyperbola, whereas Charles Law shows a linear relationship between volume and temperature when plotted on a graph.
In conclusion, Boyle’s Law and Charles Law share similarities in terms of being empirical gas laws and relating to the pressure, volume, and temperature of gases. However, they differ in their specific relationships and graphical representations. Understanding these laws is crucial for studying and predicting the behavior of gases in various scenarios.
Advantages of using Charles Law Worksheet with Answers PDF
The Charles Law Worksheet with Answers PDF offers a number of advantages for students and educators alike. This type of worksheet allows students to practice and reinforce their understanding of Charles’s Law, which states that the volume of a gas is directly proportional to its temperature, assuming constant pressure.
One of the main advantages of using a worksheet with answers in PDF format is that it provides immediate feedback to students. As they complete the worksheet, they can easily check their answers against the provided solutions. This allows them to identify any misconceptions or errors in their understanding of Charles’s Law and make corrections right away.
- Convenient: The PDF format of the worksheet allows students to access it easily on their computers, tablets, or smartphones. They can work on the worksheet anytime and anywhere, as long as they have an internet connection. This convenience promotes independent learning and flexibility in studying.
- Effective Study Tool: The Charles Law Worksheet with Answers PDF serves as an effective study tool for students. By practicing and reviewing the concepts and calculations related to Charles’s Law, students can solidify their understanding and improve their problem-solving skills. This type of active learning promotes deeper comprehension and retention of the material.
- Time-Saving: The worksheet with answers saves time for both students and educators. Students can quickly assess their progress and understanding by checking their answers against the provided solutions. Educators can also use it as a time-saving tool for grading and evaluating their students’ comprehension of Charles’s Law.
- Variety and Adaptability: With the Charles Law Worksheet with Answers PDF, educators can easily customize and adapt the worksheet to meet the specific needs of their students. They can modify the questions, add additional exercises, or create different versions of the worksheet to cater to different skill levels or learning objectives. This flexibility allows educators to create engaging and challenging assignments that cater to the individual needs of their students.
In conclusion, using a Charles Law Worksheet with Answers in PDF format offers numerous advantages for both students and educators. It provides immediate feedback, promotes independent learning, saves time, and allows for customization and adaptability. By incorporating this type of worksheet into their teaching practice, educators can enhance their students’ understanding and mastery of Charles’s Law.