Chemistry is a fascinating branch of science that deals with the composition, structure, properties, and changes of matter. Chapter 11 of a chemistry textbook often focuses on particular topics, such as chemical reactions, stoichiometry, and equilibrium. In this article, we will explore the answers to the assessment questions found in Chapter 11 of a chemistry textbook.
One of the important concepts covered in Chapter 11 is chemical reactions. Chemical reactions involve the conversion of reactants into products, and understanding the reaction stoichiometry is crucial. The assessment questions in this chapter may ask students to balance chemical equations, determine the limiting reactant, or calculate the percent yield of a reaction.
Another key topic covered in Chapter 11 is stoichiometry, which deals with the quantitative relationships between reactants and products in a chemical reaction. Assessment questions may require students to calculate the molar ratios between different substances in a reaction, determine the amount of product formed from a given amount of reactant, or solve for the mass of a reactant needed to produce a desired amount of product.
Lastly, Chapter 11 may also cover the concept of equilibrium, which occurs when the rate of the forward reaction is equal to the rate of the reverse reaction. Assessment questions may test students’ understanding of the factors that affect equilibrium, such as temperature, pressure, and concentration. They may also ask students to calculate equilibrium constant values or predict the direction in which a reaction will proceed under certain conditions.
Chemistry Chapter 11 Assessment Answers

Chemistry Chapter 11 covers the topic of chemical reactions. At the end of the chapter, students are often given an assessment to test their understanding of the concepts discussed. This assessment typically consists of a series of questions that require students to apply their knowledge of chemical reactions.
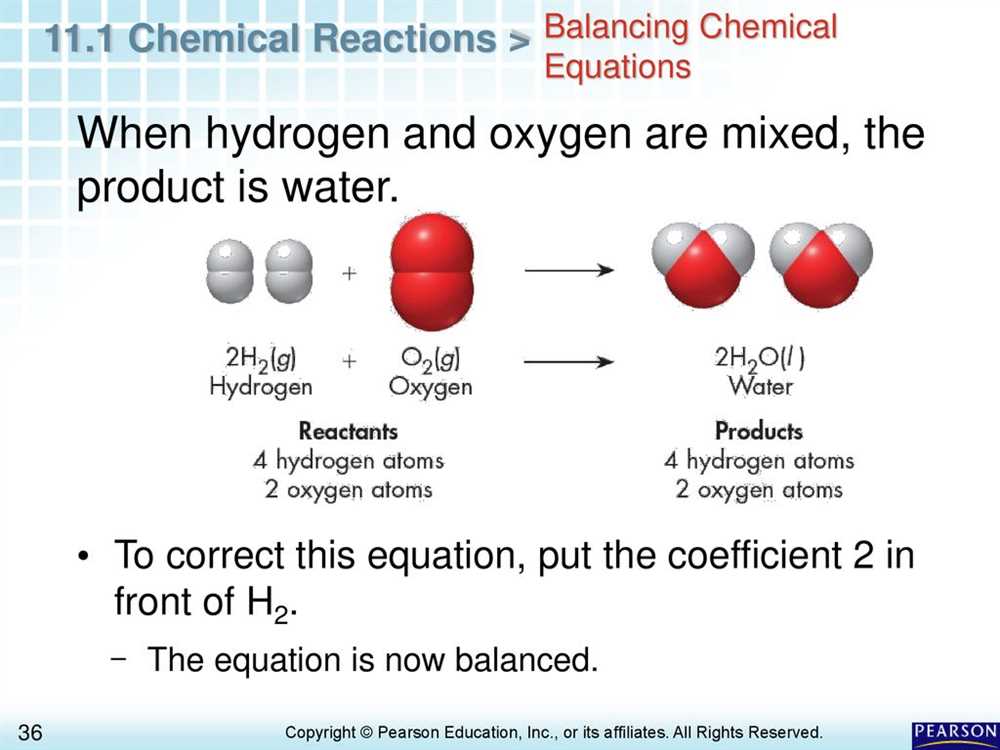
One common question on the assessment asks students to balance chemical equations. Balancing equations is a fundamental skill in chemistry, as it allows us to understand the stoichiometry of reactions and determine the relative amounts of reactants and products. Students are typically given an unbalanced chemical equation and must use their knowledge of chemical formulas and conservation of mass to balance the equation.
Another type of question on the assessment asks students to classify reactions based on their characteristics. For example, students may be asked to identify whether a given reaction is a synthesis, decomposition, combustion, single displacement, or double displacement reaction. In order to answer these questions, students must understand the different types of reactions and the general patterns that occur.
Additionally, the assessment may include questions that require students to calculate the quantities of reactants or products in a chemical reaction. This involves using stoichiometry to convert between moles of substances and applying the concept of the limiting reactant. Students will need to understand how to use the coefficients in a balanced equation to determine the mole ratios, as well as how to use molar mass to convert between moles and grams.
In conclusion, the Chemistry Chapter 11 assessment covers a range of topics related to chemical reactions. Students can expect to be tested on their ability to balance equations, classify reactions, and perform stoichiometric calculations. It is important for students to review and practice these concepts in order to succeed on the assessment.
Understanding the Basics: What is Chemistry?
In the realm of science, chemistry is a fundamental discipline that explores the composition, structure, behavior, and properties of matter. It is a branch of physical science that deals with the study of how atoms and molecules interact and combine to form various substances. Chemistry plays a crucial role in our everyday lives, as it is involved in everything from the food we eat to the medicines we take and the materials we use.
At its core, chemistry seeks to understand the building blocks of matter and their transformations. It examines the nature of elements, compounds, and mixtures, as well as the chemical reactions that occur when substances interact. Chemists study the properties of different substances, such as their physical states, boiling points, melting points, reactivity, and solubility.
Atoms and molecules
– In chemistry, atoms are the smallest units of matter that retain the chemical properties of an element. They consist of a nucleus, made up of protons and neutrons, surrounded by electrons in energy levels.
– Molecules, on the other hand, are composed of two or more atoms bonded together. They can be either elements or compounds.
– Understanding the structure and behavior of atoms and molecules is essential in chemistry, as it helps explain the properties and behavior of substances.
Chemical reactions
– Chemical reactions involve the rearrangement of atoms to form new substances. They occur when atoms or molecules collide with enough energy to break and form bonds.
– Reactants are the starting materials in a chemical reaction, while products are the substances formed as a result of the reaction.
– Chemical equations are used to represent chemical reactions, with reactants on the left side and products on the right side.
Applications of chemistry
– Chemistry has a wide range of applications in various fields, including medicine, agriculture, environmental science, materials science, and biochemistry.
– In medicine, chemistry is crucial for drug discovery and development, as well as understanding how substances interact with the human body.
– In agriculture, chemistry plays a role in developing fertilizers, pesticides, and understanding soil composition.
– In environmental science, chemistry helps study the impact of pollutants and find ways to mitigate environmental damage.
Overall, chemistry serves as the foundation for understanding the world around us at a molecular level. By studying the interactions and properties of matter, chemists can discover new substances, develop innovative materials, and contribute to advancements in various scientific fields.
Exploring Chapter 11: An Overview
Chapter 11 of the Chemistry textbook delves into the topic of chemical reactions. In this chapter, we will explore the fundamental concepts of reactions, including the different types of reactions, how they occur, and how to balance chemical equations. Understanding chemical reactions is crucial as it allows us to predict the products of a reaction and analyze the changes taking place on a molecular level.
In this chapter, we will begin by examining the different types of reactions, such as combination reactions, decomposition reactions, displacement reactions, and double displacement reactions. Each type of reaction involves different reactants and products, and understanding their characteristics will enable us to identify and classify reactions in the future.
One important aspect of chemical reactions is balancing chemical equations. When a reaction occurs, atoms are rearranged to form new substances. Balancing the equation ensures that the same number and type of atoms are present on both sides of the reaction. We will learn how to balance equations by adjusting coefficients, which represent the number of molecules or formula units involved in the reaction. Through practice and understanding the concept of conservation of mass, balancing equations will become easier and allow us to accurately represent the reactants and products involved.
Overall, Chapter 11 is an essential part of the Chemistry curriculum as it provides the foundation for understanding chemical reactions. By exploring the types of reactions and learning how to balance chemical equations, we will gain a deeper understanding of the molecular world and be able to apply these concepts to various real-life scenarios.
Key Concepts: Elements, Compounds, and Mixtures
The study of chemistry involves understanding the fundamental building blocks of matter, which are elements. An element is a pure substance that cannot be broken down into simpler substances by chemical means. Each element is made up of atoms that have the same number of protons in their nuclei. There are currently 118 known elements, each with its own unique set of properties and characteristics. Elements are represented by symbols, such as H for hydrogen and O for oxygen, which are listed in the periodic table.
Compounds, on the other hand, are substances that are formed when two or more elements combine chemically. In a compound, the atoms of different elements are held together by chemical bonds. The properties of a compound are different from those of its constituent elements. For example, sodium (Na) is a highly reactive metal, while chlorine (Cl) is a poisonous gas. However, when sodium and chlorine combine to form sodium chloride (NaCl), a compound known as table salt, the properties of the resulting compound are completely different. Sodium chloride is a white crystalline solid that is essential for human health and used in various industries.
Mixtures, unlike compounds, are combinations of two or more substances that are physically intermingled but not chemically combined. Mixtures can be either homogeneous or heterogeneous. In a homogeneous mixture, the components are evenly distributed throughout, resulting in a uniform and consistent appearance. Examples of homogeneous mixtures include air and saltwater. In contrast, a heterogeneous mixture has visibly distinct phases or regions with different compositions. Examples of heterogeneous mixtures include oil and water and salad dressing.
In conclusion, understanding the concepts of elements, compounds, and mixtures is crucial in the study of chemistry. Elements are the fundamental building blocks of matter, compounds are formed when elements chemically combine, and mixtures are combinations of substances that are physically intermingled. By studying these concepts, chemists can better understand the properties and behaviors of different substances, as well as how they interact and react with one another.
The Periodic Table: A Guide to Elements
The periodic table is a valuable tool used by chemists to organize and understand the properties of different elements. It is a visual representation of the building blocks of matter, where each element is assigned a unique symbol and sorted into specific groups and periods based on its atomic number and electron configuration.
At first glance, the periodic table may seem complex, with its rows and columns filled with symbols and numbers. However, it provides crucial information about the elements, such as their atomic mass, atomic number, and chemical symbol. The arrangement of elements in the periodic table allows scientists to make predictions about an element’s properties based on its position.
The periodic table is divided into groups, which are vertical columns, and periods, which are horizontal rows. Elements within the same group have similar properties because they have the same number of valence electrons, which are the electrons in the outermost energy level of an atom. Elements in the same period have the same number of energy levels.
- Metals: The majority of elements on the periodic table are metals, which are located on the left side and middle of the table. Metals are typically shiny, malleable, ductile, and good conductors of heat and electricity.
- Nonmetals: Nonmetals are located on the right side of the periodic table. They have properties opposite to metals, such as being brittle, poor conductors of heat and electricity, and dull in appearance.
- Metalloids: Metalloids, also known as semimetals, are located along the staircase-shaped line that separates metals from nonmetals. They exhibit properties of both metals and nonmetals.
In addition to the physical properties, the periodic table also provides information about an element’s chemical reactivity. For example, elements on the far left side of the periodic table, such as alkali metals, are highly reactive and easily lose electrons, while elements on the far right side, such as noble gases, are stable and nonreactive.
In conclusion, the periodic table is a powerful tool that allows chemists to understand the properties and behavior of elements. By organizing elements based on their atomic number and electron configuration, the periodic table provides a roadmap for studying and predicting the behavior of elements in chemical reactions. Its structure and arrangement provide valuable insights into the world of chemistry.
Chemical Equations and Reactions
In chemistry, chemical equations are used to represent the changes that occur during a chemical reaction. They describe the reactants, or the substances that are being combined or broken apart, and the products, which are the resulting substances. Chemical equations are written using formulas and chemical symbols to represent the different elements and compounds involved in the reaction.
Chemical reactions can be classified into different types based on the changes that occur. One common type of reaction is a synthesis reaction, where two or more substances combine to form a single product. Another type is a decomposition reaction, where a single compound breaks apart into two or more simpler substances. Other types include combustion reactions, where a substance reacts with oxygen to produce carbon dioxide and water, and redox reactions, where there is a transfer of electrons between reactants.
Chemical equations follow certain rules to ensure that they are balanced. The principle of conservation of mass states that matter cannot be created or destroyed in a chemical reaction, so the number of atoms of each element must be the same on both sides of the equation. To achieve this, coefficients are used to balance the equation, indicating the number of molecules or atoms involved in the reaction.
Key Terms:
- Chemical equations: representations of chemical reactions using formulas and chemical symbols.
- Reactants: the substances that undergo changes in a chemical reaction.
- Products: the substances that are formed as a result of a chemical reaction.
- Synthesis reaction: a type of reaction where two or more substances combine to form a single product.
- Decomposition reaction: a type of reaction where a single compound breaks apart into simpler substances.
- Combustion reaction: a reaction where a substance reacts with oxygen to produce carbon dioxide and water.
- Redox reaction: a reaction where there is a transfer of electrons between reactants.
- Conservation of mass: the principle that matter cannot be created or destroyed in a chemical reaction.
- Coefficients: numbers used to balance a chemical equation.
Understanding chemical equations and reactions is fundamental to studying chemistry. By analyzing and interpreting these equations, scientists are able to understand the changes that occur during a reaction and make predictions about the products and properties of substances. Balancing chemical equations allows for accurate representation of the stoichiometry, or the relative amounts of reactants and products, in a reaction. This knowledge is essential for a wide range of applications, from industrial processes to environmental studies.