
Understanding gas laws is an essential part of studying chemistry. These laws allow scientists to make predictions and calculations about the behavior of gases under different conditions. To help students practice applying these laws, teachers often provide worksheets with questions and problems. In order to check their answers, students need an answer key that provides the correct solutions.
The chemistry gas laws worksheet answer key is a valuable resource for students. It allows them to check their work and ensure that they have understood and applied the concepts correctly. The answer key is typically organized by topic, with each section focusing on a different gas law. For example, students might find sections on Boyle’s Law, Charles’s Law, and the combined gas law. Each section includes a set of questions or problems, along with the corresponding answers.
Using the answer key, students can compare their answers to the correct solutions. If they find that their answers are incorrect, they can go back and review the relevant gas law to understand their mistake. The answer key also serves as a study tool, as it allows students to see the correct steps and calculations for each problem. This can help them improve their problem-solving skills and build a stronger understanding of gas laws.
Overall, the chemistry gas laws worksheet answer key is an essential tool for students studying gas laws in chemistry. It provides them with a way to check their work, identify any mistakes, and improve their understanding of the subject. By using the answer key effectively, students can enhance their learning experience and excel in their chemistry studies.
Overview

The Chemistry gas laws worksheet answer key is a comprehensive resource that provides students with the solution to various problems related to gas laws. These gas laws describe the behavior of gases under different conditions, such as pressure, volume, and temperature. The worksheet answer key allows students to check their answers and understand the steps involved in solving the problems.
The gas laws covered in the worksheet answer key include Boyle’s law, Charles’s law, Gay-Lussac’s law, and the combined gas law. Boyle’s law states that the pressure and volume of a gas are inversely proportional to each other, while Charles’s law states that the volume and temperature of a gas are directly proportional. Gay-Lussac’s law states that the pressure and temperature of a gas are directly proportional.
- Boyle’s law: P1V1 = P2V2
- Charles’s law: V1/T1 = V2/T2
- Gay-Lussac’s law: P1/T1 = P2/T2
- Combined gas law: P1V1/T1 = P2V2/T2
The gas laws worksheet answer key provides step-by-step solutions to problems that involve these gas laws. It helps students understand the concepts and calculations involved in solving the problems. The key also includes explanations and examples to further enhance students’ understanding of gas laws and their applications in real-world situations.
Overall, the Chemistry gas laws worksheet answer key is a valuable tool for students studying gas laws in chemistry. It allows them to practice solving problems and check their answers, reinforcing their understanding of the subject and helping them succeed in their studies.
What are gas laws?
The study of gases is an important branch of chemistry. In order to understand and predict the behavior of gases, scientists have developed a set of laws known as the gas laws. These laws describe the relationships between pressure, volume, temperature, and the amount of gas in a system.
Boyle’s Law: This law states that the pressure of a gas is inversely proportional to its volume, at constant temperature. In other words, if the volume of a gas decreases, its pressure will increase, and vice versa.
Charles’s Law: This law states that the volume of a gas is directly proportional to its temperature, at constant pressure. As the temperature of a gas increases, its volume will also increase, and as the temperature decreases, so will its volume.
Gay-Lussac’s Law: This law states that the pressure of a gas is directly proportional to its temperature, at constant volume. When the temperature of a gas increases, its pressure will also increase, and when the temperature decreases, so will its pressure.
Combined, these laws form the ideal gas law, which can be used to calculate the properties of gases in various situations. The ideal gas law is expressed as PV = nRT, where P is the pressure, V is the volume, n is the number of moles of gas, R is the ideal gas constant, and T is the temperature.
Understanding gas laws is essential for a wide range of applications, including the design of chemical processes, the study of atmospheric phenomena, and the development of new materials and technologies. By applying these laws, scientists and engineers can accurately predict and control the behavior of gases in different conditions.
The Importance of Gas Laws in Chemistry
Gas laws are a fundamental aspect of chemistry that help us understand and describe the behavior of gases. They are derived from experimental observations and mathematical models, and provide valuable insights into the properties of gases, including pressure, volume, temperature, and the number of gas particles.
One of the most well-known gas laws is Boyle’s Law, which states that the pressure and volume of a gas are inversely proportional at constant temperature. This law helps us understand how the volume of a gas changes with changes in pressure, and is crucial in various applications such as scuba diving, where changes in pressure affect the volume of air in a diver’s lungs.
Another important gas law is Charles’s Law, which states that the volume and temperature of a gas are directly proportional at constant pressure. This law helps us understand how gases expand or contract with changes in temperature, and is essential in applications such as weather forecasting, where changes in temperature affect the volume of air in the atmosphere.
Gas laws also play a crucial role in the field of gas chemistry, where they help scientists predict and manipulate the behavior of gases in various chemical reactions. For example, the Ideal Gas Law combines Boyle’s Law, Charles’s Law, and Avogadro’s Law to describe the behavior of gases under any conditions. This law allows chemists to calculate the number of gas particles, volume, temperature, or pressure of a gas, given the other three variables.
In summary, gas laws are essential in chemistry as they provide a framework to understand and describe the behavior of gases. They help us make predictions, explain experimental observations, and manipulate the properties of gases in various applications. By studying gas laws, chemists can gain a deeper understanding of the physical and chemical properties of gases, and apply this knowledge in diverse fields such as medicine, environmental science, and industry.
Boyle’s Law
Boyle’s Law, also known as the Boyle-Mariotte Law, is one of the fundamental gas laws in chemistry. It describes the relationship between the pressure and volume of a gas at constant temperature. According to Boyle’s Law, the pressure of a gas is inversely proportional to its volume when temperature remains constant.
The mathematical expression of Boyle’s Law is as follows:
P1V1 = P2V2
This equation indicates that as the pressure of a gas increases, its volume decreases, and vice versa, as long as the temperature remains constant. In simpler terms, if a gas is compressed, its pressure increases, and if a gas expands, its pressure decreases.
Boyle’s Law is particularly important when working with gases, as it helps to explain the behavior of gases under different conditions. It is commonly used in various applications, such as determining the volume of gases in a chemical reaction or understanding the behavior of gases in different containers.
Overall, Boyle’s Law provides valuable insights into the relationship between pressure and volume in gases, allowing scientists and chemists to better understand and predict the behavior of gases in various scenarios.
Definition and Formula
The study of gas laws is an important aspect of chemistry, as it helps us understand the behavior of gases under different conditions. Gas laws describe the relationships between pressure, volume, temperature, and the amount of gas present. These laws allow us to predict and calculate various properties of gases.
One of the fundamental gas laws is Boyle’s Law, which states that for a fixed amount of gas at a constant temperature, the pressure and volume of the gas are inversely proportional. Mathematically, this can be represented as:
P1V1 = P2V2
Where P1 and P2 are the initial and final pressures, and V1 and V2 are the initial and final volumes.
Another important gas law is Charles’ Law, which states that the volume of a gas is directly proportional to its temperature, assuming constant pressure. The mathematical representation of Charles’ Law is:
V1/T1 = V2/T2
Here, V1 and V2 are the initial and final volumes, and T1 and T2 are the initial and final temperatures.
These are just two examples of gas laws, and there are several other laws that describe the behavior of gases. Understanding these laws and their mathematical representations is essential for solving problems related to gas properties.
Example problems with solutions
In this section, we will solve a few example problems related to gas laws in chemistry. These problems will help you understand how to apply the gas laws to real-life situations. Let’s dive in!
Problem 1: Boyle’s Law
Given: Initial volume (V1) = 3 L, Initial pressure (P1) = 2 atm, Final volume (V2) = 2 L
To find: Final pressure (P2)
Solution:
- Apply Boyle’s Law equation: P1V1 = P2V2
- Substitute the given values: 2 atm * 3 L = P2 * 2 L
- Simplify the equation: 6 atm = P2 * 2 L
- Divide both sides by 2 L: P2 = 6 atm / 2 L
- Calculate the final pressure: P2 = 3 atm
Therefore, the final pressure (P2) in this problem is 3 atm.
Problem 2: Ideal Gas Law

Given: Pressure (P) = 1 atm, Volume (V) = 4 L, Temperature (T) = 300 K, Gas constant (R) = 0.0821 L·atm/(K·mol)
To find: Number of moles (n)
Solution:
- Apply the Ideal Gas Law equation: PV = nRT
- Substitute the given values: 1 atm * 4 L = n * 0.0821 L·atm/(K·mol) * 300 K
- Simplify the equation: 4 atm·L = n * 24.63 L·atm/(K·mol)
- Divide both sides by 24.63 L·atm/(K·mol): n = 4 atm·L / 24.63 L·atm/(K·mol)
- Calculate the number of moles: n = 0.162 mol
Therefore, the number of moles (n) in this problem is 0.162 mol.
These example problems demonstrate the application of gas laws in solving various situations. Practice solving more problems to strengthen your understanding of gas laws and their use in chemistry.
Charles’s Law
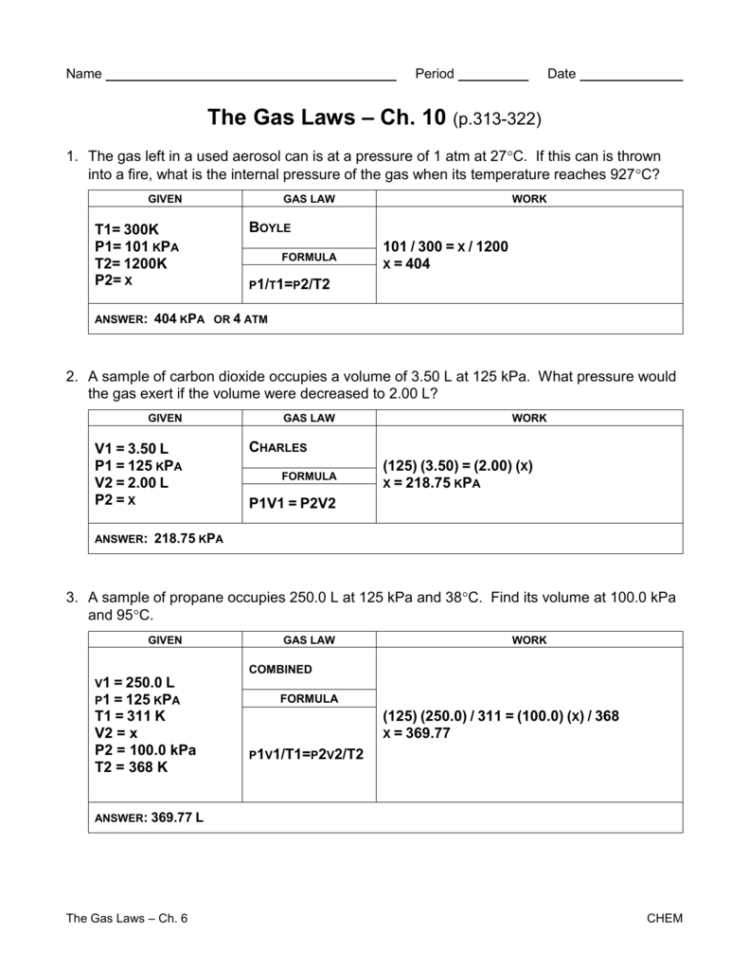
Charles’s Law is one of the fundamental gas laws in chemistry that describes the relationship between the volume and temperature of a gas. According to Charles’s Law, at constant pressure, the volume of a gas is directly proportional to its absolute temperature. This means that as the temperature of a gas increases, its volume will also increase, and as the temperature decreases, the volume will decrease.
The mathematical representation of Charles’s Law can be written as V1/T1 = V2/T2, where V1 and V2 are the initial and final volumes of the gas, and T1 and T2 are the initial and final temperatures of the gas respectively. This equation shows that if the temperature of a gas doubles, its volume will also double, assuming the pressure remains constant.
Charles’s Law helps chemists and scientists understand the behavior of gases under different conditions. It is particularly useful in applications such as determining the volume of a gas at a specific temperature or predicting the change in volume of a gas with changing temperature. Furthermore, Charles’s Law is closely related to other gas laws, such as Boyle’s Law and Gay-Lussac’s Law, which together form the ideal gas law.
Definition and Formula
The gas laws are a set of mathematical relationships that describe the behavior of gases under different conditions. They provide a way to quantify the properties of gases such as pressure, volume, temperature, and amount of substance. One of the fundamental gas laws is Boyle’s law, which states that the volume of a gas is inversely proportional to its pressure, assuming temperature and amount of substance remain constant.
The formula for Boyle’s law is:
- P1V1 = P2V2
Where P1 and P2 are the initial and final pressures, and V1 and V2 are the initial and final volumes. This formula shows that as the pressure of a gas increases, its volume decreases, and vice versa, as long as the temperature and amount of substance remain constant. This relationship is known as the pressure-volume relationship.
Another important gas law is Charles’s law, which states that the volume of a gas is directly proportional to its temperature, assuming pressure and amount of substance remain constant.
The formula for Charles’s law is:
- V1/T1 = V2/T2
Where V1 and V2 are the initial and final volumes, and T1 and T2 are the initial and final temperatures in Kelvin. This formula shows that as the temperature of a gas increases, its volume also increases, and vice versa, as long as the pressure and amount of substance remain constant. This relationship is known as the temperature-volume relationship.
Application of Charles’s Law
In the study of gases, one important relationship is Charles’s Law, which states that the volume of a given amount of gas is directly proportional to its temperature, assuming that pressure and moles of gas remain constant. This relationship can be expressed mathematically as V1/T1 = V2/T2, where V1 and T1 are the initial volume and temperature, and V2 and T2 are the final volume and temperature.
Charles’s Law finds numerous applications in various fields of science and industry. One such application is in the design and operation of hot air balloons. A hot air balloon operates on the principle that hot air is less dense than cold air. As the temperature of the air inside the balloon increases, its volume expands, making the balloon buoyant and capable of lifting off the ground. This application of Charles’s Law allows hot air balloons to soar through the sky.
Another practical application of Charles’s Law is in the automotive industry. In the engines of cars and other vehicles, the combustion of fuel generates high temperatures that can cause the air inside the engine to expand. By applying Charles’s Law, engineers can design engines and ventilation systems that can accommodate the expansion of air and prevent damage to the engine. This application helps ensure the efficiency and longevity of automotive engines.
Charles’s Law is also relevant in laboratory settings, particularly in experiments involving gases. Scientists use this gas law to determine the relationship between temperature and volume and to conduct accurate measurements. By manipulating the temperature of gases in a controlled environment, researchers can observe and analyze the effects of temperature changes on various properties of gases, such as pressure and solubility.
In conclusion, Charles’s Law has a wide range of applications in various industries and scientific fields. From the operation of hot air balloons to the design of automotive engines, this gas law provides crucial insights into the behavior of gases and allows for the development of efficient and safe systems.