Understanding the atomic structure is crucial in comprehending the fundamental building blocks of matter. Chemthink Atomic Structure is a platform that offers interactive exercises and simulations to help students grasp the concept of atomic structure. With the help of Chemthink Atomic Structure, students have the opportunity to explore the world of atoms, electrons, and protons, and learn about their interactions and properties.
One of the key questions that Chemthink Atomic Structure seeks to answer is: How are atoms structured? Through engaging visuals and interactive exercises, students can uncover the answers to this fundamental question. They can examine the arrangement of protons, neutrons, and electrons within an atom, and understand how these components contribute to the overall chemical properties of an element.
Chemthink Atomic Structure also provides answers to questions such as: How do electrons occupy energy levels? Students can explore the concept of electron energy levels and understand how electrons distribute themselves within an atom’s energy levels in accordance with the Aufbau principle. By interacting with simulations, students can visualize the filling of energy levels and gain a deeper understanding of electron configurations.
Furthermore, Chemthink Atomic Structure addresses the question: How do atoms interact with one another? Students can delve into the concept of chemical bonding and explore the forces that hold atoms together to form molecules. By understanding the interactions between atoms and the role of electrons in chemical bonding, students can make sense of various types of bonding, such as covalent, ionic, and metallic bonds.
In conclusion, Chemthink Atomic Structure is an interactive platform that offers answers to key questions about atomic structure, electron configurations, and chemical bonding. Through engaging visuals and simulations, students can deepen their understanding of these fundamental concepts and develop a solid foundation in chemistry.
Atomic Structure: Exploring the Building Blocks of Matter
Atomic structure refers to the arrangement and organization of particles that make up an atom. Atoms are the basic building blocks of matter, and understanding their structure is essential in the field of chemistry. By studying atomic structure, scientists can gain insights into the properties and behavior of different elements, as well as the reactions they undergo.
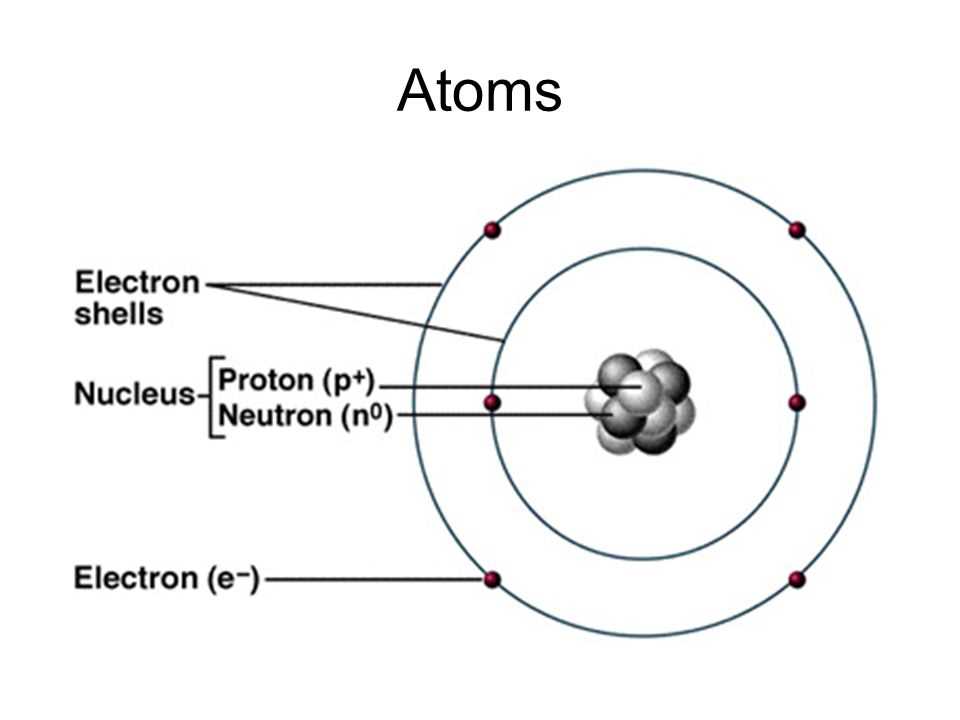
An atom consists of three main components: protons, neutrons, and electrons. Protons and neutrons are located in the nucleus, which is the central part of the atom. Protons have a positive charge, while neutrons have no charge. Electrons, on the other hand, orbit around the nucleus in specific energy levels or shells. They have a negative charge and are responsible for the chemical properties and bonding behavior of atoms.
The number of protons in an atom determines its atomic number, which identifies the element. For example, hydrogen atoms have one proton, while carbon atoms have six. The sum of protons and neutrons in the nucleus gives the atomic mass of an atom. Electrons are typically equal in number to protons, resulting in an overall neutral charge for the atom.
Further exploration of atomic structure has revealed that protons and neutrons are made up of even smaller particles called quarks. These elementary particles have different combinations of charges, creating the different types of quarks. The discovery of quarks and the study of their interactions have deepened our understanding of atomic structure and the fundamental forces that govern matter.
| Component | Charge | Location | Mass |
|---|---|---|---|
| Proton | Positive (+) | Nucleus | ~1 atomic mass unit |
| Neutron | No charge (neutral) | Nucleus | ~1 atomic mass unit |
| Electron | Negative (-) | Orbitals | ~0.0005 atomic mass unit |
Overall, atomic structure provides the foundation for understanding the behavior, properties, and interactions of atoms and elements. By delving into the intricacies of atomic structure, scientists continue to uncover the secrets of matter and contribute to advancements in various fields of science and technology.
Breaking down the basics

Understanding the atomic structure is fundamental to grasping the concepts of chemistry. At the most basic level, atoms are the building blocks of matter. They are incredibly small, with a nucleus at the center that contains protons and neutrons, and electrons orbiting around the nucleus. The number of protons defines the element, while the number of neutrons can vary within the same element, creating isotopes.
Atomic number is the number of protons in an atom. It determines the element’s identity. For example, hydrogen has an atomic number of 1, while carbon has an atomic number of 6. Atomic mass, on the other hand, is the sum of protons and neutrons in an atom. It provides an understanding of the atom’s mass compared to a standard reference point.
Electrons orbit the nucleus in specific energy levels or shells. The closest shell to the nucleus can hold a maximum of 2 electrons, while the second shell can hold up to 8. This organization of electrons allows elements to form chemical bonds and participate in reactions. The arrangement of electrons within an atom is represented by an electron configuration, which follows certain rules and principles.
Additionally, it is important to understand the concept of valence electrons. These are the electrons in the outermost shell of an atom, and they have a significant influence on an element’s chemical behavior. Elements with similar numbers of valence electrons tend to have similar chemical properties and can form similar types of compounds.
Understanding Atomic Particles
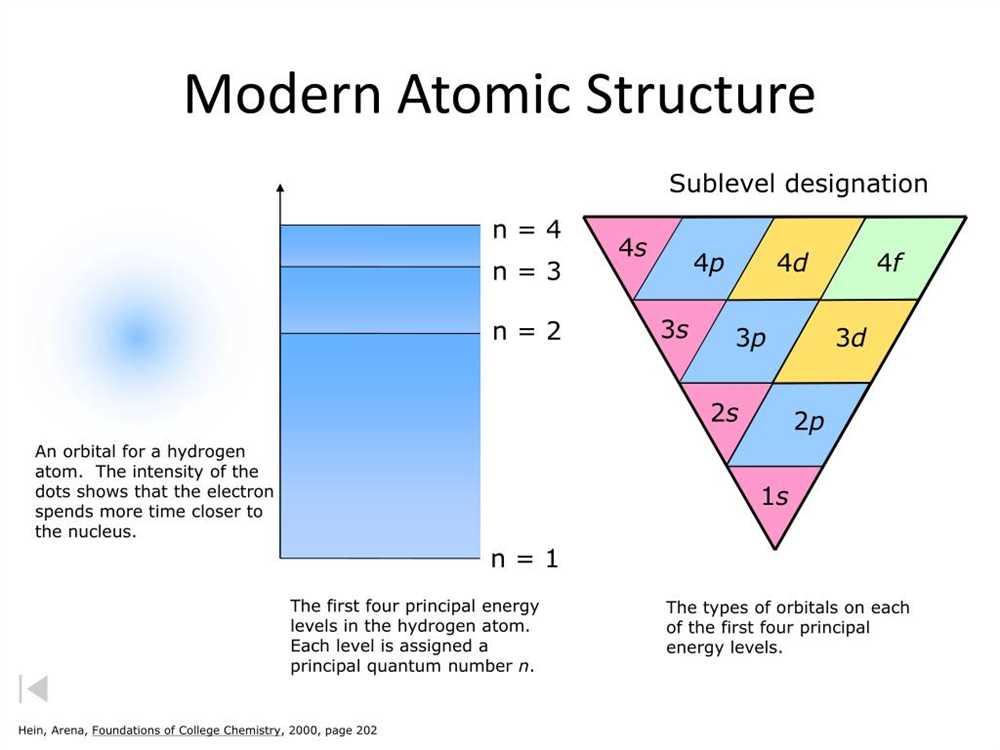
The study of atomic structure is essential to understanding the behavior and properties of matter. At the most fundamental level, everything in the universe is composed of atoms, which are made up of smaller particles called subatomic particles. This exploration of atomic particles allows scientists to uncover the intricacies of matter and explain phenomena that occur on a microscopic scale.
Atom: An atom is the basic unit of matter. It consists of a nucleus, which contains protons and neutrons, surrounded by electrons in energy levels or shells. The nucleus carries a positive charge, while electrons carry a negative charge. The number of protons determines the element and its atomic number, while the number of neutrons can vary, giving rise to different isotopes of an element.
Proton: Protons are positively charged particles found in the nucleus of an atom. They have a mass of approximately 1 atomic mass unit (amu) and are responsible for determining the atomic number of an element. The number of protons in an atom identifies its unique characteristics and defines its place on the periodic table.
Neutron: Neutrons are uncharged particles found in the nucleus of an atom. They have a mass similar to that of protons (approximately 1 amu) but carry no charge. Neutrons help stabilize the nucleus by counteracting the repulsive forces between positively charged protons. The number of neutrons can vary among atoms of the same element, leading to the formation of isotopes with different masses.
Electron: Electrons are negatively charged particles that orbit the nucleus of an atom. They are much smaller and lighter than protons and neutrons, with a mass of only about 0.0005 amu. Electrons occupy specific energy levels or shells, and their distribution determines the chemical behavior of an atom. Electrons can gain or lose energy, causing them to move to different energy levels or participate in chemical reactions.
The understanding of these atomic particles and their interactions allows scientists to explain the behavior of matter, the formation of compounds, and the principles governing chemical reactions. By studying the composition and arrangement of atoms, scientists continue to unlock the secrets of the universe and develop technologies that shape our world.
Importance of Understanding Atomic Structure
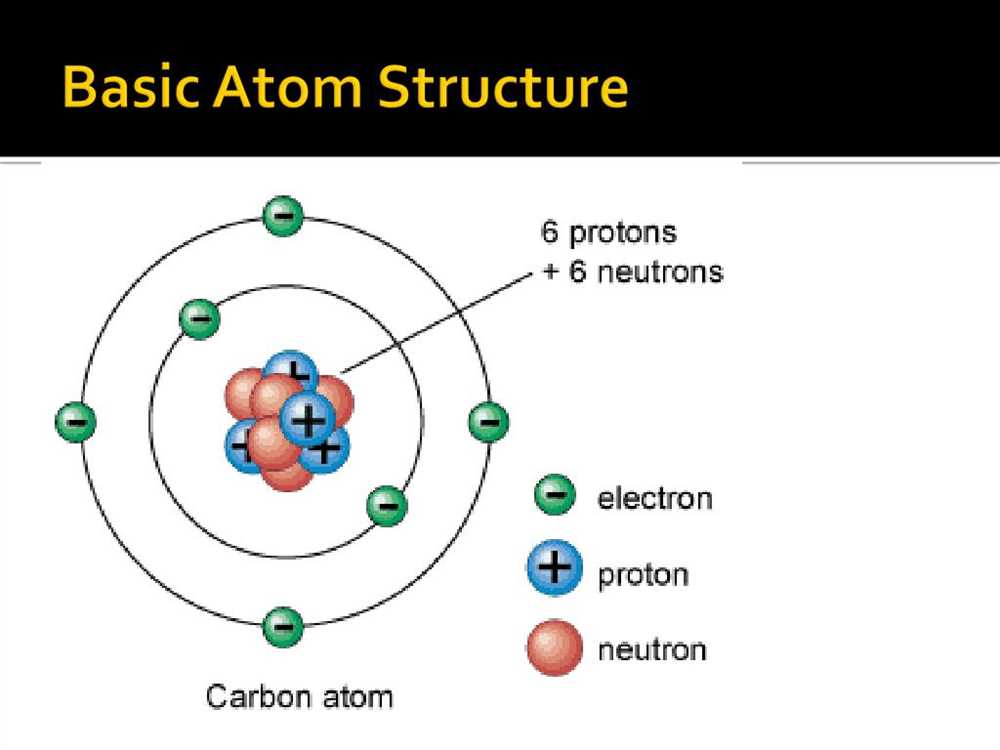
The study of atomic structure is crucial in various scientific fields, such as chemistry and physics, as it forms the foundation for our understanding of matter and its interactions. A strong understanding of atomic structure enables scientists to predict and explain the behavior of different elements, compounds, and reactions, allowing for the development of new materials and technologies.
1. Fundamental Building Block: Atoms are the fundamental building blocks of matter. They consist of a nucleus that contains positively charged protons and uncharged neutrons, surrounded by negatively charged electrons. Understanding the arrangement and behavior of these particles helps us understand the properties and characteristics of different elements and compounds, as well as how they interact with each other.
2. Chemical Reactions: Atomic structure is crucial for understanding chemical reactions. It helps us predict which elements will bond with each other and how they will form compounds. By understanding the arrangement of electrons in an atom, scientists can determine the stability of different elements and their ability to gain, lose, or share electrons, which ultimately determines their reactivity and chemical properties.
3. Energy Levels and Spectra: The study of atomic structure also plays a vital role in understanding energy levels and spectra. Electrons in atoms can exist in different energy levels or orbitals, and when they absorb or release energy, they emit distinct spectra of light. This knowledge is essential in fields such as spectroscopy, where scientists analyze the interaction of light with matter to study the composition and structure of substances.
4. Nanotechnology and Materials Science: Understanding atomic structure is crucial in the field of nanotechnology and materials science. Manipulating and arranging atoms at the atomic scale allows scientists to create new materials with unique properties and functionalities. By understanding the behavior of atoms and their interactions, scientists can design materials with desired properties, such as increased strength, flexibility, or conductivity, leading to advancements in various industries and technologies.
Overall, a thorough understanding of atomic structure is essential for scientists across various disciplines. It provides the basis for understanding the properties and behavior of matter, predicting and explaining chemical reactions, analyzing spectra, and developing new materials and technologies. Without this understanding, many scientific advancements and technological innovations would not be possible.
Chemthink Atomic Structure Simulation
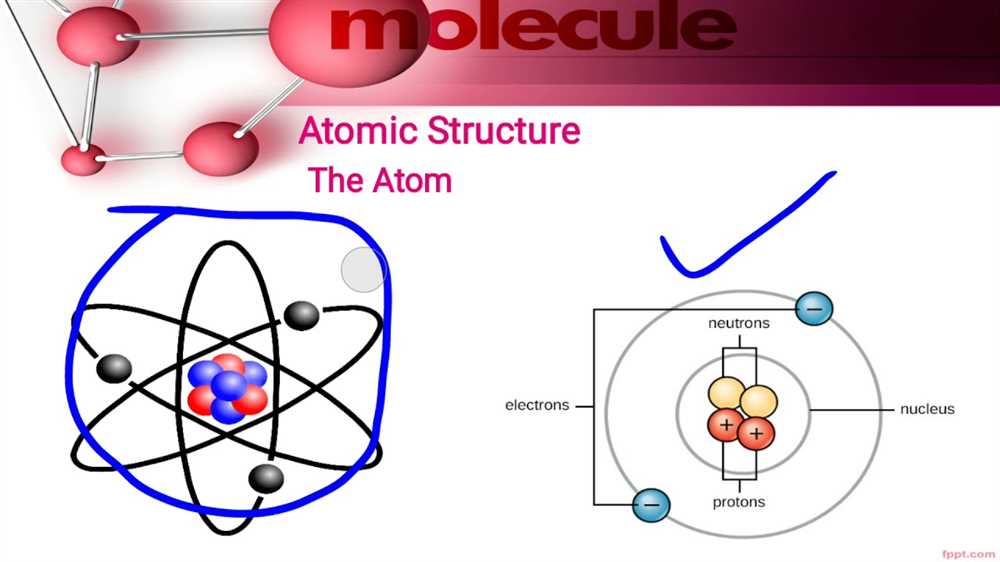
The Chemthink Atomic Structure simulation is an interactive and educational tool that allows users to explore the fundamental concepts of atomic structure. Through a series of activities and simulations, users can manipulate and observe atoms to understand their composition, behavior, and the principles of atomic structure.
One of the key features of the Chemthink Atomic Structure simulation is the ability to build atoms using different elements from the periodic table. Users can select elements and arrange their protons, neutrons, and electrons to create atoms of varying sizes and atomic numbers. This hands-on approach allows users to visualize the relationship between atomic structure and an element’s properties.
The simulation also provides a detailed explanation of the different parts of an atom and their functions. Users can learn about the role of protons and neutrons in the nucleus, as well as the arrangement of electrons in energy levels or shells. They can explore the concept of valence electrons and understand how they contribute to chemical bonding and reactivity.
In addition to building atoms, the Chemthink Atomic Structure simulation also allows users to explore atomic models and theories throughout history. Users can learn about the contributions of scientists such as John Dalton, J.J. Thomson, and Niels Bohr, and understand how their discoveries shaped our understanding of atomic structure. They can also compare and contrast different models, such as the Bohr model and the quantum mechanical model, to see how our understanding of atoms has evolved over time.
Overall, the Chemthink Atomic Structure simulation is a valuable tool for students and educators alike. It provides an engaging and interactive way to learn about the fundamental principles of atomic structure and helps build a strong foundation in chemistry.
Exploring the interactive tool
The Chemthink atomic structure interactive tool is designed to help students understand the fundamental concepts and principles of atomic structure. By using this interactive tool, students can explore various aspects of atoms, such as their composition, arrangement, and behavior.
One key feature of this tool is the ability to build atoms by adding protons, neutrons, and electrons. Students can choose different numbers of these particles and observe how they affect the overall properties of the atom. This hands-on approach allows students to see the direct relationship between the number of subatomic particles and the resulting atomic structure.
The interactive tool also provides a visual representation of the electron cloud model, which shows the probability of finding an electron in different regions around the nucleus. This model helps students understand the concept of electron orbitals and how they contribute to the overall behavior of atoms.
In addition to building atoms and exploring the electron cloud model, the interactive tool offers interactive quizzes and activities. These quizzes test students’ understanding of the material and provide immediate feedback, allowing them to review and reinforce their knowledge.
The Chemthink atomic structure interactive tool is a valuable resource for students studying atomic structure. It offers a hands-on and visual approach to learning and helps students develop a deeper understanding of the fundamental principles of atoms and their behavior.