If you are looking for answers to the Collision Theory Gizmo, this article will provide you with the information you need. The Collision Theory Gizmo is a simulation tool that allows students to explore the effects of temperature, concentration, and surface area on the rate of chemical reactions.
The Collision Theory Gizmo provides a hands-on approach to learning about collision theory, which is a fundamental concept in chemistry. By manipulating the variables in the simulation, students can observe how changing factors such as temperature or concentration can affect the rate of collisions between particles and therefore the rate of reaction.
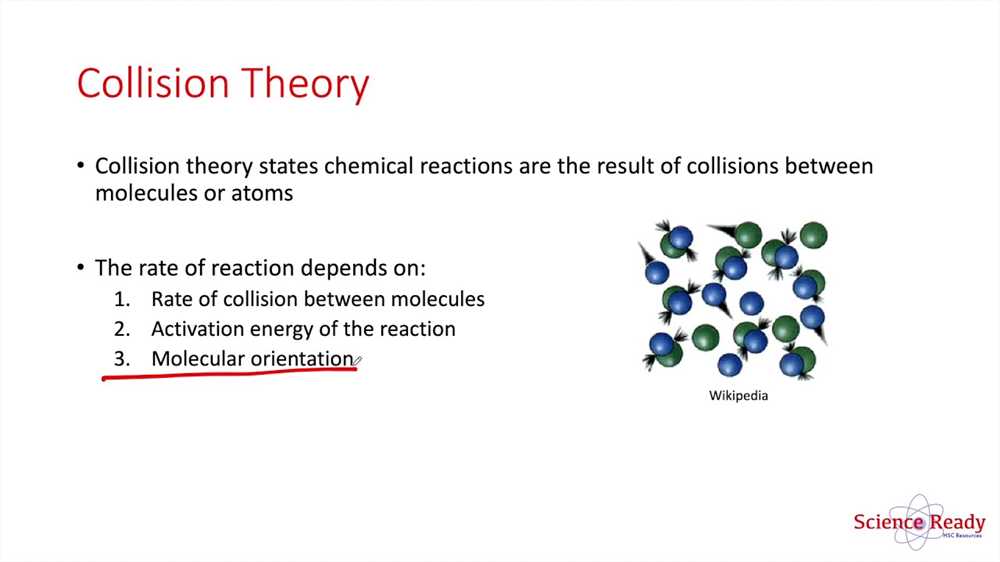
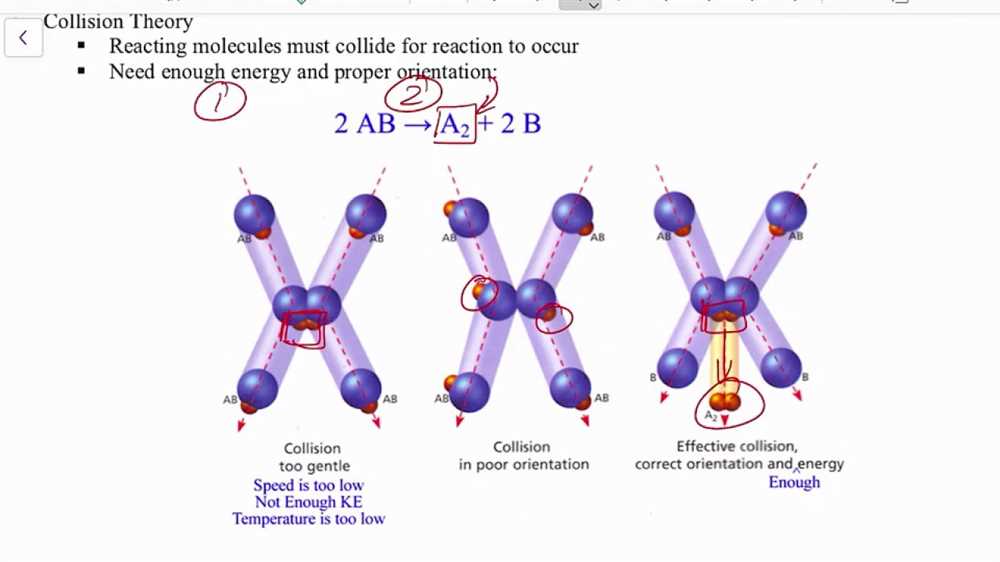
In order to find answers to the Collision Theory Gizmo, it is important to understand the basic principles of collision theory. According to collision theory, in order for a chemical reaction to occur, particles must collide with sufficient energy and in the correct orientation. Increasing the temperature or concentration of reactants can increase the number of collisions, while increasing the surface area of a solid reactant can increase the likelihood of collisions between particles.
By using the Collision Theory Gizmo, students can explore these principles in a virtual laboratory setting. The Gizmo allows students to manipulate the variables and observe the resulting changes in the rate of reaction. This hands-on approach to learning can help students develop a deeper understanding of collision theory and how it relates to chemical reactions.
What is the Collision Theory Gizmo?
The Collision Theory Gizmo is an online interactive simulation tool that allows students to explore and understand the principles behind collision theory in chemistry. It is a valuable educational resource for learning about the factors that affect the rate of chemical reactions.
Collision theory states that for a chemical reaction to occur, particles must collide with sufficient energy and in the correct orientation. By using the Collision Theory Gizmo, students can experiment with different factors and observe how they influence the rate of reaction.
This interactive tool allows students to vary parameters such as temperature, concentration, and particle size to see how these factors impact the probability and effectiveness of collisions. The Gizmo also provides real-time graphs and data analysis tools, which allow students to visualize and interpret the results of their experiments.
With the Collision Theory Gizmo, students can develop a deeper understanding of the key concepts of collision theory and how they relate to the kinetics of chemical reactions. It provides a hands-on and engaging platform for students to explore and discover the factors that affect reaction rates, helping them to build a solid foundation in chemistry.
Overview of the Gizmo
The Collision Theory Gizmo is an interactive simulation that allows students to explore the concept of collisions between particles. It is designed to help students understand the factors that influence rates of chemical reactions. With this Gizmo, students can manipulate different variables and observe how they affect the rate of reaction.
The Gizmo provides a virtual environment where students can conduct experiments and gather data. It presents a scenario where two particles, represented by colored circles, are moving in a confined space. By adjusting parameters such as temperature, concentration, and particle size, students can see how these factors impact the frequency and effectiveness of collisions.
The simulation is accompanied by a data table that records the number of collisions and successful collisions. This allows students to compare the effects of different variables on the rate of reaction. Through this hands-on approach, students can develop a deeper understanding of the Collision Theory and its application in the real world.
The Collision Theory Gizmo is a valuable tool for chemistry education as it provides an interactive and visual way for students to explore concepts related to chemical reactions. By experimenting with different variables, students can see firsthand how factors such as temperature and concentration affect the rate of reaction. This helps them build a strong foundation in the principles of collision theory and prepares them for more complex topics in chemistry.
How Does the Gizmo Work?
The Collision Theory Gizmo is a virtual lab that simulates different scenarios of chemical reactions and allows students to explore the factors that affect the speed of these reactions. It utilizes the principles of collision theory, which states that for a chemical reaction to occur, particles must collide with sufficient energy and in the correct orientation.
The Gizmo provides a simulation environment where students can manipulate various variables such as temperature, concentration, and surface area to observe their effects on reaction rate. Students can choose different reactants and adjust their concentrations to see how it influences the number of collisions and the rate of reaction. They can also adjust the temperature to see how it affects the velocity of particles and their collision energy.
The Gizmo visually displays the reactant particles as colored dots in a container. When the simulation starts, the particles move randomly, and collisions between them are shown as arrows. By analyzing the data provided by the Gizmo, students can make predictions and draw conclusions about the impact of different variables on reaction rate.
The Gizmo enhances understanding and learning by providing a hands-on experience that allows students to interact with the concepts of collision theory in a virtual lab setting. It provides a safe and controlled environment for experimentation, allowing students to make observations and empirical connections between variables and reaction rate. By manipulating the Gizmo’s parameters and analyzing the resulting data, students are able to develop a deeper understanding of the factors that influence the speed of chemical reactions.
Importance of Collision Theory in Chemistry
The collision theory is a fundamental concept in chemistry that explains the reaction rates and the factors that influence them. It states that for a chemical reaction to occur, the reactant particles must collide with the appropriate energy and orientation. This concept is crucial in understanding the kinetics of chemical reactions and predicting their rates.
One of the main applications of collision theory is in the field of reaction kinetics. By analyzing the collision rates and the energy and orientation requirements, scientists can determine the rate at which a chemical reaction will take place. This information is essential for various industries, such as pharmaceuticals, where knowing the reaction rate is crucial for optimizing production processes and developing new drugs.
In addition to reaction kinetics, collision theory also helps in understanding the factors that influence the reaction rates. These factors include temperature, concentration, surface area, and the presence of a catalyst. By studying the effects of these factors on the collision frequency and energy, scientists can manipulate them to control and enhance the rates of chemical reactions.
Understanding collision theory is also important in environmental chemistry. By knowing the factors that influence reaction rates, scientists can predict the rates at which pollutants degrade in the atmosphere or in water bodies. This knowledge aids in developing strategies to mitigate pollution and improve the quality of the environment.
In conclusion, collision theory plays a vital role in chemistry by providing the basic framework for understanding reaction kinetics and predicting reaction rates. It helps scientists optimize production processes, develop new drugs, and mitigate environmental pollution. Understanding the factors that influence collision rates and energy is crucial for advancing our knowledge in various fields of chemistry.
Understanding Collision Theory
The Collision Theory is a fundamental concept in chemistry that explains the rate at which chemical reactions occur. It is based on the idea that for a reaction to occur, particles (atoms, molecules, or ions) must collide with each other with enough energy and proper orientation. This theory helps chemists understand the factors that influence the reaction rate and how to control it.
Key Concepts:
- Collision: Collision occurs when particles come into contact with each other.
- Energy: For a reaction to occur, particles must collide with sufficient energy. The minimum energy required for a successful collision is called the activation energy.
- Orientation: The particles must also collide in the correct orientation. This means that their relative positions and angles should be favorable for reaction to occur.
According to the Collision Theory, increasing the concentration of reactants, temperature, or pressure increases the reaction rate. This is because a higher concentration means more collisions, a higher temperature increases the energy of collisions, and increased pressure leads to more frequent collisions. Additionally, using a catalyst can lower the activation energy required for a reaction, thereby increasing the reaction rate.
In conclusion, understanding the principles of the Collision Theory is crucial for chemists to design and control chemical reactions. By manipulating the factors that influence collision frequency and energy, scientists can optimize reaction conditions and improve the efficiency of chemical processes.
Relevance of Collision Theory in Chemical Reactions
The collision theory is a fundamental concept in understanding and predicting chemical reactions. It provides a framework for explaining the factors that influence the rate of a reaction, as well as the conditions required for a reaction to occur. By considering the interactions between particles in a reaction, the collision theory helps scientists to design and optimize chemical processes.
According to the collision theory, for a chemical reaction to occur, the reacting molecules must collide with sufficient energy and with the correct orientation. The energy of the collision must overcome the activation energy barrier, which is the minimum energy required for the reaction to proceed. The orientation of the colliding molecules is important because it determines whether the reactants will form the desired products or not.
Factors influencing the rate of a reaction:
- Concentration of reactants: In general, a higher concentration of reactants increases the frequency of collisions and therefore the rate of reaction.
- Temperature: Increasing the temperature provides more kinetic energy to the reactant molecules, leading to more frequent and energetic collisions.
- Surface area: A larger surface area exposes more reactant particles to collisions, increasing the chances of a successful reaction.
- Catalysts: Catalysts lower the activation energy barrier, allowing more reactant molecules to possess enough energy to initiate the reaction. They do not participate in the reaction itself and are not consumed in the process.
Applications of the collision theory:
The collision theory has significant practical applications in various fields, including:
- Industrial chemistry: Understanding the factors that influence reaction rates helps in designing efficient chemical processes. By adjusting the concentration, temperature, and other parameters, scientists can optimize reaction conditions and increase the production rate.
- Pharmaceuticals: The collision theory helps in the development of new drugs and medications. By studying the reactions between drug molecules and target receptors, scientists can design drugs with the desired properties and enhance their efficacy.
- Environmental science: The collision theory is used to understand and model chemical reactions that occur in the atmosphere, such as the formation of ozone and the breakdown of pollutants. This knowledge helps in predicting environmental impacts and designing strategies for pollution control.
In conclusion, the collision theory provides a valuable framework for understanding the factors that influence chemical reactions. By considering the energy and orientation of colliding molecules, scientists can predict reaction rates, optimize reaction conditions, and design new chemical processes in various fields.
Frequently Asked Questions about Collision Theory Gizmo Answers
In the context of studying collision theory, students often have questions about finding answers to the Collision Theory Gizmo. Here are some frequently asked questions and their answers:
1. Where can I find the answers to the Collision Theory Gizmo?
The answers to the Collision Theory Gizmo can usually be found in the accompanying teacher’s guide or answer key. These resources are commonly provided by the teacher or can sometimes be accessed online. It’s important to note that understanding the concepts and principles behind collision theory is more valuable than simply finding the answers. So, make sure to review the materials and seek clarification from your teacher when needed.
2. Can I use the Collision Theory Gizmo answers to cheat on my homework or tests?
No, using the Collision Theory Gizmo answers to cheat on your homework or tests is not recommended. The purpose of assigning these tasks is to assess your understanding and application of the concepts. Cheating not only undermines your own learning but also goes against academic integrity. It is crucial to put in the effort to learn and understand the material instead of relying on shortcuts.
3. I’m having trouble with the Collision Theory Gizmo. Where can I get help?
If you are having trouble with the Collision Theory Gizmo or any other aspect of your studies, don’t hesitate to seek help. Start by reaching out to your teacher or instructor, as they are there to guide you through the learning process. They can provide explanations, clarifications, and additional resources to support your understanding. Additionally, consider studying with classmates or utilizing online resources such as tutorials or forums dedicated to chemistry education.
How to Access Collision Theory Gizmo Answers?
If you are looking for answers to the Collision Theory Gizmo, there are a few different ways you can access them. The Collision Theory Gizmo is an interactive simulation that allows students to explore the concepts of chemical reactions and collision theory. It is often used in science classrooms to help students understand how factors such as temperature, concentration, and surface area affect the rate of a chemical reaction.
One way to access the Collision Theory Gizmo answers is by asking your teacher or instructor. They may have the answers or be able to guide you to a resource that can provide them. Another option is to search for online forums or discussion boards where other students or educators may have shared the answers. These forums can be a great resource for finding help and connecting with others who are studying the same material.
Table:
| Methods to access Collision Theory Gizmo answers: |
|---|
| Ask your teacher or instructor. |
| Search for online forums or discussion boards. |
| Use online resources or study guides. |
| Collaborate with classmates or study groups. |
You can also try using online resources or study guides that may provide answers or explanations. These resources can be found on educational websites, textbooks, or even through online tutoring services. Finally, you can collaborate with your classmates or form study groups to work together on finding the answers. This can be a helpful way to share ideas, discuss concepts, and find solutions to challenging questions.
In conclusion, there are multiple ways to access Collision Theory Gizmo answers. Whether through your teacher, online forums, study guides, or collaboration with classmates, it’s important to utilize the resources available to help improve your understanding of the concept and enhance your learning experience.