Collision theory is an essential concept in understanding the science behind chemical reactions and physical phenomena. It delves into the factors that influence the rate and outcome of collisions between particles, providing valuable insights into the mechanisms that drive various processes.
For students and enthusiasts seeking a deeper understanding of collision theory, the Collision Theory Gizmo is a valuable tool. This interactive simulation allows users to explore different scenarios and manipulate variables to observe the effects on collision rates and outcomes. However, finding the answers to the Gizmo’s questions can sometimes be challenging, especially for those looking for a comprehensive resource.
In this article, we provide a solution to this challenge by offering a Collision Theory Gizmo answers PDF. This resource compiles the answers to the questions posed in the Gizmo, allowing users to quickly and easily access the information they need for a comprehensive understanding of collision theory. Whether you are a student studying for an exam or a curious individual seeking knowledge, this Collision Theory Gizmo answers PDF will prove to be an invaluable reference.
Understanding the gizmo
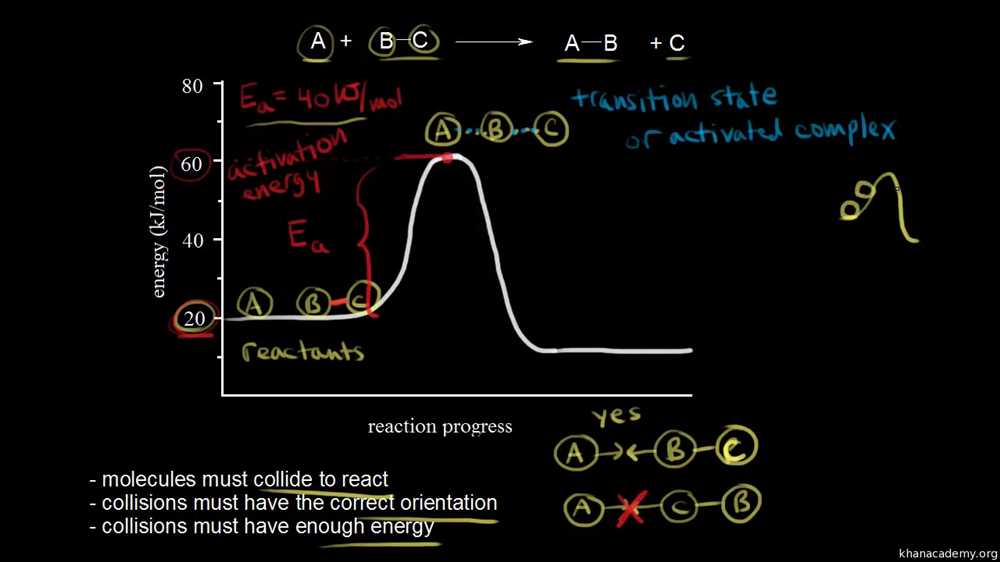
In order to understand the Collision Theory Gizmo, it is important to first grasp the concept of collision theory itself. Collision theory is a fundamental principle in chemistry that relates to the rates of chemical reactions. It states that in order for a chemical reaction to occur, particles must collide with each other with enough energy and in the correct orientation.
The Gizmo is an interactive tool that allows users to explore different factors that affect the rate of chemical reactions based on collision theory. It provides a simulation environment where users can adjust various parameters and observe the effects on reaction rates.
One of the key elements of the Gizmo is the ability to control the temperature of the reaction. Temperature is an important factor in collision theory as it directly affects the kinetic energy of particles. By increasing the temperature, users can observe how the reaction rate changes as more particles have enough energy to overcome the activation energy barrier and collide effectively.
Another important parameter that can be adjusted in the Gizmo is the concentration of reactants. Collision theory states that a higher concentration of reactants leads to more frequent collisions, increasing the likelihood of successful reactions. By changing the concentration, users can observe how the reaction rate is affected.
Overall, the Collision Theory Gizmo provides a valuable tool for understanding the principles of collision theory and how different factors influence the rate of chemical reactions. By allowing users to experiment with temperature and concentration, it helps to solidify the concepts and make them more tangible.
The Importance of Collision Theory in Chemistry
Collision theory is a fundamental concept in chemistry that explains how chemical reactions occur. It provides insight into the factors that influence the rate of a reaction and helps chemists predict the outcome of different reactions. By understanding collision theory, scientists can design more efficient and effective reactions, develop new drugs, and improve various industrial processes.
Key principles of collision theory:
- Molecular collisions: According to collision theory, chemical reactions occur when molecules collide with sufficient energy and proper orientation. This collision between reactant molecules leads to the formation of new products.
- Reaction rate: Collision theory explains that the rate of a chemical reaction depends on the frequency of molecular collisions. The more collisions that occur, the faster the reaction.
- Activation energy: Collision theory also introduces the concept of activation energy, which is the minimum energy required for a collision to result in a reaction. Molecules must have enough energy to overcome this activation energy barrier in order to react.
By applying collision theory, chemists can manipulate reaction conditions to control the rate and outcome of a reaction. They can adjust factors such as temperature, concentration, pressure, and catalysts to increase the number of collisions and provide the necessary energy for successful reactions. This knowledge is crucial in the development of new drugs, where chemists must optimize reaction conditions to produce the desired compounds.
In industrial processes, collision theory plays a vital role in improving efficiency. Understanding the factors that influence reaction rates allows scientists to design reactors and optimize reaction conditions to maximize production and minimize waste. By applying collision theory, industries can reduce energy consumption, decrease operating costs, and improve overall productivity.
Applying collision theory to reactions
Collision theory is a fundamental concept in chemistry that helps us understand the factors that influence the rate of chemical reactions. According to this theory, for a reaction to occur, the reactant particles must collide with sufficient energy and in the correct orientation. By understanding and applying collision theory, scientists can manipulate reaction conditions to enhance the rate of desired reactions.
Reactant concentration: One of the key factors that affects the rate of a reaction is the concentration of the reactants. According to collision theory, increasing the concentration of reactant particles increases the number of collisions and therefore the rate of reaction. This is because a higher concentration means there are more reactant particles available to collide with one another, leading to a higher chance of successful reactions.
Temperature: Another important factor in collision theory is temperature. As temperature increases, the kinetic energy of the reactant particles also increases. This means that the particles move faster and collide with greater force. According to collision theory, an increase in temperature increases the frequency and energy of collisions, resulting in a higher rate of reaction.
Catalysts: Catalysts are substances that can speed up the rate of a chemical reaction without being consumed in the reaction. They work by providing an alternative reaction pathway with a lower activation energy. Collision theory explains this phenomenon by stating that catalysts increase the chances of successful collisions between reactant particles by providing a surface for them to adhere to and facilitating the breaking and formation of bonds.
Conclusion: Collision theory provides a framework for understanding the factors that influence the rate of chemical reactions. By manipulating reactant concentration, temperature, and using catalysts, scientists can apply collision theory to enhance the rate of desired reactions in various practical applications, ranging from industrial processes to pharmaceutical development.
Exploring the Answers to Collision Theory Gizmo
The Collision Theory Gizmo is an interactive simulation tool that allows students to explore the concept of collision theory in chemistry. By manipulating variables such as temperature, surface area, and concentration, students can investigate how these factors affect the rate of a chemical reaction. With the ability to visualize and collect data, the Gizmo provides a hands-on approach to understanding collision theory.
One of the questions that students often have when using the Collision Theory Gizmo is, “How does temperature affect the rate of a chemical reaction?” By increasing the temperature, students can observe that the particles move faster and collide more frequently. This leads to an increase in the rate of the reaction. Conversely, when the temperature is decreased, the particles move slower and collide less often, resulting in a decrease in the reaction rate. This experiment helps students to understand the relationship between temperature and reaction rate, and how it relates to collision theory.
The Gizmo also allows students to explore how surface area affects reaction rate. By changing the size of the simulated reactant particles, students can observe that smaller particles have a larger surface area available for collisions. This increased surface area leads to a higher reaction rate because more particles are available for successful collisions. Conversely, larger particles have a smaller surface area and therefore fewer collisions, resulting in a lower reaction rate. This experiment helps students understand the importance of surface area in collision theory and its impact on reaction rate.
In conclusion, the Collision Theory Gizmo provides a valuable tool for exploring the answers to questions related to collision theory in chemistry. Through hands-on experimentation and visualization, students can gain a deeper understanding of how factors such as temperature and surface area affect reaction rate. By engaging with the Gizmo, students can develop a solid foundation in collision theory, which is essential for understanding chemical reactions and their kinetics.
Key concepts and principles
In the study of collision theory, there are several key concepts and principles that are important to understand. These concepts help to explain how particles interact and ultimately determine the outcome of a collision.
Particles: When studying collision theory, it is important to consider the characteristics of the particles involved. These particles can be atoms, molecules, or ions, and their size, shape, and energy all play a role in determining the likelihood of a collision occurring.
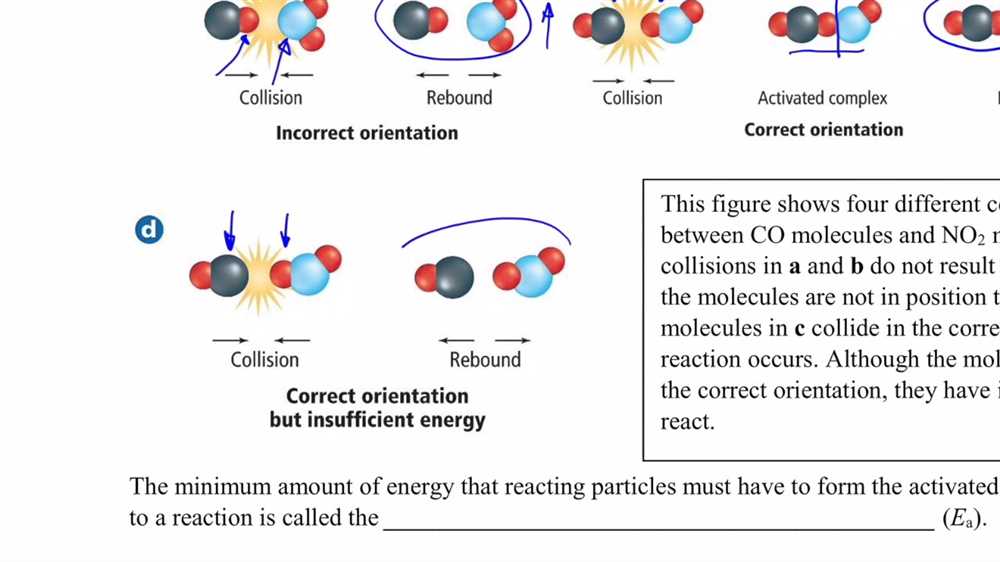
Collisions: Collisions can be classified as either effective or ineffective. An effective collision occurs when particles collide with enough energy and in the correct orientation to cause a reaction. Ineffective collisions, on the other hand, do not have enough energy or the proper orientation to result in a reaction.
Activation energy: Activation energy is the minimum amount of energy required for a collision to be effective. If the colliding particles do not possess enough energy to overcome this barrier, the reaction will not occur. Activation energy can be thought of as a sort of “threshold” that must be crossed in order for a reaction to take place.
Reaction rate: The reaction rate is a measure of how quickly a reaction occurs. In collision theory, the reaction rate is determined by the frequency of effective collisions. The more often particles collide and have enough energy to overcome the activation energy, the faster the reaction will proceed.
Overall, collision theory provides a framework for understanding the factors that influence the likelihood and speed of chemical reactions. By considering the particles involved, the type of collisions that occur, the energy required for a reaction, and the rate at which collisions take place, scientists can gain insights into the underlying principles of chemical reactions.
Using the collision theory gizmo for educational purposes
The collision theory gizmo is an excellent educational tool that helps students understand and visualize the concept of collision theory in chemistry. This interactive simulation allows students to experiment and observe how different factors, such as temperature, concentration, and surface area, affect the rate of chemical reactions.
One benefit of using the collision theory gizmo is that it provides a hands-on approach to learning. Instead of simply reading about collision theory in a textbook, students can actively engage with the gizmo and manipulate the variables to see the direct impact on reaction rates. This helps to deepen their understanding and make the abstract concepts more concrete.
Temperature: With the collision theory gizmo, students can easily see the effect of temperature on reaction rates. By increasing the temperature, they can observe how the particles move faster and collide more frequently, resulting in a higher reaction rate. This visual representation helps students grasp the concept of thermal energy and its influence on chemical reactions.
Concentration: The gizmo allows students to alter the concentration of the reactants and witness how it affects reaction rates. By increasing the concentration, students can see an increase in the number of particles available for collisions, leading to more frequent successful collisions and a faster reaction rate. This hands-on exploration reinforces the relationship between concentration and reaction rates.
Surface area: Another key aspect of collision theory is the surface area of the reactants. By manipulating the surface area in the gizmo, students can observe how a larger surface area leads to more frequent collisions and a faster reaction rate. This visual demonstration helps students understand why finely divided substances react more rapidly than those in bulk form.
In conclusion, the collision theory gizmo is a valuable educational tool that enhances students’ understanding of collision theory in chemistry. By allowing students to actively engage with the simulation and manipulate variables, the gizmo helps make abstract concepts more tangible and promotes a deeper understanding of chemical reactions and their influencing factors.