
The solubility of a substance in a solvent can be influenced by the presence of another ion that is already present in the solution. This phenomenon is known as the common ion effect and it is an important concept in chemistry.
When a solute is dissolved in a solvent, it dissociates into its constituent ions. The solubility of a compound is determined by the equilibrium between the dissolved ions and the undissolved solid. When an ion that is already present in the solution is added, it can shift this equilibrium and affect the solubility of the compound.
For example, let’s consider the solubility of calcium fluoride (CaF2) in water. Calcium fluoride dissociates into calcium ions (Ca2+) and fluoride ions (F–). If we add sodium fluoride (NaF) to the solution, it will increase the concentration of fluoride ions. This increase in the concentration of fluoride ions can shift the equilibrium towards the undissolved solid and decrease the solubility of calcium fluoride.
The common ion effect can also be used to control the solubility of substances in various industrial processes. It is often employed in the field of pharmaceuticals, where precise control over solubility is crucial for drug formulation. By carefully manipulating the concentration of common ions, scientists and engineers can optimize the solubility of drugs and improve their efficacy.
What is the common ion effect on solubility?
The common ion effect on solubility refers to the phenomenon where the solubility of a salt is decreased when a solution already contains an ion that is also present in the salt. This effect occurs due to the principle of Le Chatelier’s principle, which states that a system at equilibrium will respond to a disturbance by shifting its equilibrium position in a way that minimizes the disturbance.
When a solution contains a common ion, the equilibrium solubility of a salt is decreased because the presence of the common ion pushes the equilibrium towards the solid state, shifting the equilibrium position. This can be explained by the decrease in the ionic product of the salt, reducing the concentration of dissolved ions in the solution.
The common ion effect can also be explained using an example. Let’s consider the solubility of silver chloride (AgCl) in water. The solubility equilibrium for this salt can be represented as:
- AgCl(s) ↔ Ag+(aq) + Cl-(aq)
When a solution contains a high concentration of chloride ions (Cl-), for example from the addition of sodium chloride (NaCl), the common ion effect comes into play. According to Le Chatelier’s principle, the equilibrium is shifted to the left, favoring the formation of solid silver chloride. This decreases the solubility of AgCl in the presence of a common ion.
In summary, the common ion effect on solubility is a phenomenon observed when a solution contains an ion that is also present in the salt being dissolved. This effect reduces the solubility of the salt by shifting the equilibrium towards the solid state, in accordance with Le Chatelier’s principle.
How does the common ion effect work?
The common ion effect is an important concept in chemistry that explains how the solubility of a substance is affected by the presence of another ion that is already present in the solution. It states that the solubility of a compound decreases when a common ion is added to the solution.
When two substances with a common ion are dissolved in a solvent, the solubility of the less soluble substance is reduced due to the common ion. This is because the common ion reduces the concentration of the less soluble substance in the solution, causing it to become less soluble.
The common ion effect can be explained using Le Chatelier’s principle. According to Le Chatelier’s principle, when a stress is applied to a system in equilibrium, the system responds by shifting in a direction that reduces the stress. In the case of the common ion effect, the presence of the common ion is the stress, and the system responds by shifting towards the side of the equilibrium where the solubility is reduced.
For example, let’s consider the solubility of calcium carbonate (CaCO3) in water. Calcium carbonate is a sparingly soluble compound, meaning it does not readily dissolve in water. However, when calcium carbonate is added to water that already contains calcium ions (common ion), the solubility of calcium carbonate decreases significantly. This is because the presence of calcium ions in the solution reduces the concentration of calcium carbonate, making it less soluble.
In summary, the common ion effect occurs when the solubility of a compound is reduced by the presence of another ion that is already present in the solution. This effect can be explained by Le Chatelier’s principle, where the system shifts towards the side of the equilibrium where the solubility is reduced in response to the presence of the common ion.
Definition of the common ion effect
The common ion effect refers to the phenomenon in which the solubility of a slightly soluble salt is decreased by the presence of a common ion in the solution. A common ion is an ion that is already present in the solution as a result of another substance that has dissolved.
When a slightly soluble salt is placed in a solution that already contains one of its constituent ions, the equilibrium of the solubility reaction is disturbed. According to Le Chatelier’s principle, the system will shift to reestablish equilibrium by reducing the solubility of the salt. This occurs because the concentration of one of the ions in the solution is already high, so the forward reaction that dissolves the salt is less favored.
The common ion effect can be demonstrated with the solubility of silver chloride (AgCl) in water. AgCl is a slightly soluble salt that dissociates into silver ions (Ag+) and chloride ions (Cl-) in solution. If a solution of silver ions is mixed with a solution of chloride ions, the concentration of both ions will increase. As a result, the solubility of AgCl will decrease, and the formation of a precipitate of AgCl will be favored.
The common ion effect has important implications in many areas of chemistry, including analytical chemistry, where it can be used to separate and identify ions in a solution. It is also relevant in environmental chemistry, as it can affect the solubility and transport of contaminants in natural waters. By understanding the common ion effect, chemists can better predict and control the solubility of various substances in solution.
Explanation of Le Chatelier’s principle
Le Chatelier’s principle states that when a system in equilibrium is subjected to a stress, it will respond in such a way as to counteract the stress and restore equilibrium. This principle is based on the observation that when changes are made to a system at equilibrium, the concentrations of reactants and products will adjust in order to maintain the equilibrium constant.
One example of Le Chatelier’s principle in action is the effect of temperature on an equilibrium reaction. According to the principle, if a reaction is exothermic (producing heat), an increase in temperature will favor the formation of the products, and if a reaction is endothermic (absorbing heat), an increase in temperature will favor the formation of the reactants.
Another example is the effect of pressure on a gaseous equilibrium reaction. If the volume of the system is decreased (increasing the pressure), the system will shift to the side with fewer moles of gas in order to reduce the pressure. On the other hand, if the volume is increased (decreasing the pressure), the system will shift to the side with more moles of gas to restore the equilibrium.
Overall, Le Chatelier’s principle provides a useful framework for predicting and understanding how changes in temperature, pressure, and concentration can impact the position of equilibrium in a chemical reaction. By considering the stress applied to the system and the response it elicits, one can gain insights into the behavior of the reaction and make informed predictions about the resulting changes in equilibrium.
Factors Influencing the Common Ion Effect
The common ion effect is a phenomenon that affects the solubility of a compound in a solution containing a common ion. The solubility of a compound is determined by factors like temperature, pressure, and the presence of other ions in the solution. However, the presence of a common ion can have a significant impact on the solubility of a compound, leading to a decrease in its solubility.
One of the primary factors that influence the common ion effect is the concentration of the common ion. According to Le Chatelier’s principle, adding more of a common ion to a solution will shift the equilibrium towards the formation of a precipitate, thereby decreasing the solubility of the compound. The higher the concentration of the common ion, the greater the impact on solubility.
Another factor that affects the common ion effect is the nature of the common ion itself. Different ions have different abilities to form insoluble salts with other ions. For example, if the common ion is a highly charged ion, it will have a stronger effect on the solubility compared to a common ion with a lower charge. Additionally, the size and complexity of the common ion can also influence its effect on solubility.
The pH of the solution is another important factor that can influence the common ion effect. Some compounds may exhibit different solubilities at different pH values due to the presence of common ions. For example, a weak acid with a conjugate base may have a higher solubility in an acidic solution compared to a basic solution due to the common ion effect.
In summary, the common ion effect is influenced by factors such as the concentration of the common ion, the nature of the common ion, and the pH of the solution. Understanding these factors is crucial in predicting and explaining the solubility behavior of compounds in the presence of common ions.
Concentration of the common ion
The concentration of the common ion plays a crucial role in determining the solubility of a compound in a solution. When a compound is added to a solution that already contains one of its ions, the solubility of the compound is affected due to the common ion effect. This effect occurs because the presence of a common ion reduces the solubility of a slightly soluble salt.
For example, let’s consider the solubility of calcium carbonate (CaCO3) in water. Calcium carbonate is a sparingly soluble salt that dissociates into calcium ions (Ca2+) and carbonate ions (CO32-). When calcium carbonate is added to a solution containing a high concentration of calcium ions, the solubility of calcium carbonate decreases. This is because the common calcium ions in the solution decrease the overall solubility of the compound, shifting the equilibrium towards the solid form.
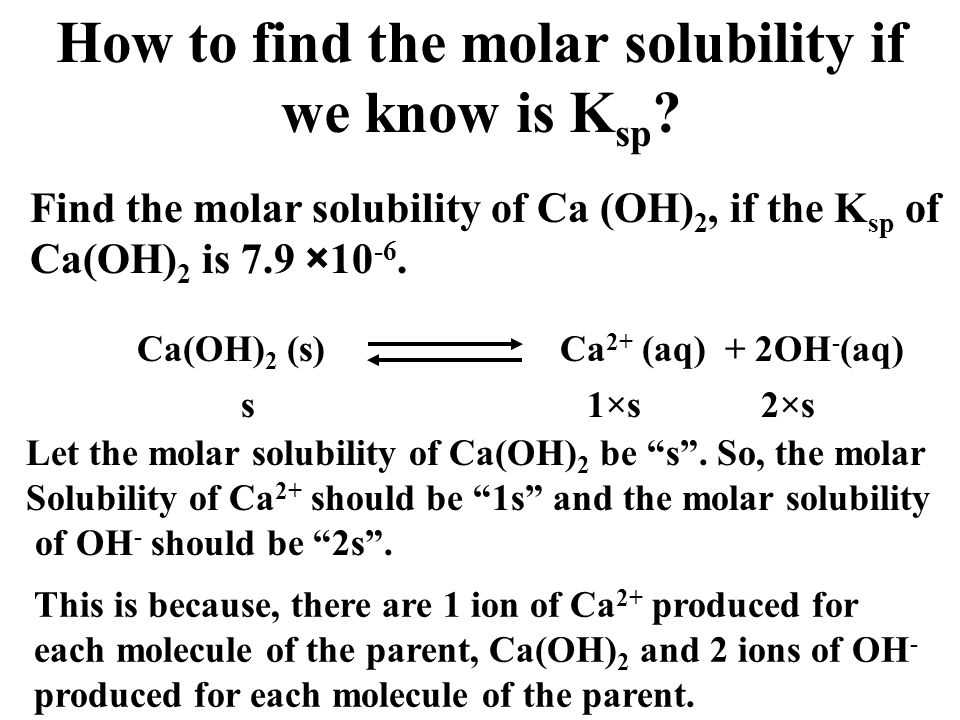
The concentration of the common ion can be calculated using the solubility product constant (Ksp) of the slightly soluble salt and the solubility of the compound. The solubility product constant is a measure of the equilibrium between the dissolved ions and the undissolved solid. By knowing the solubility of the compound and the Ksp value, the concentration of the common ion can be determined.
Understanding the concentration of the common ion is important in many aspects of chemistry. It helps predict the solubility of compounds in various solutions, as well as the formation of precipitates. By controlling the concentration of the common ion, the solubility of a compound can be manipulated, making it a valuable tool in chemical reactions and processes.
Solubility Product Constant (Ksp)
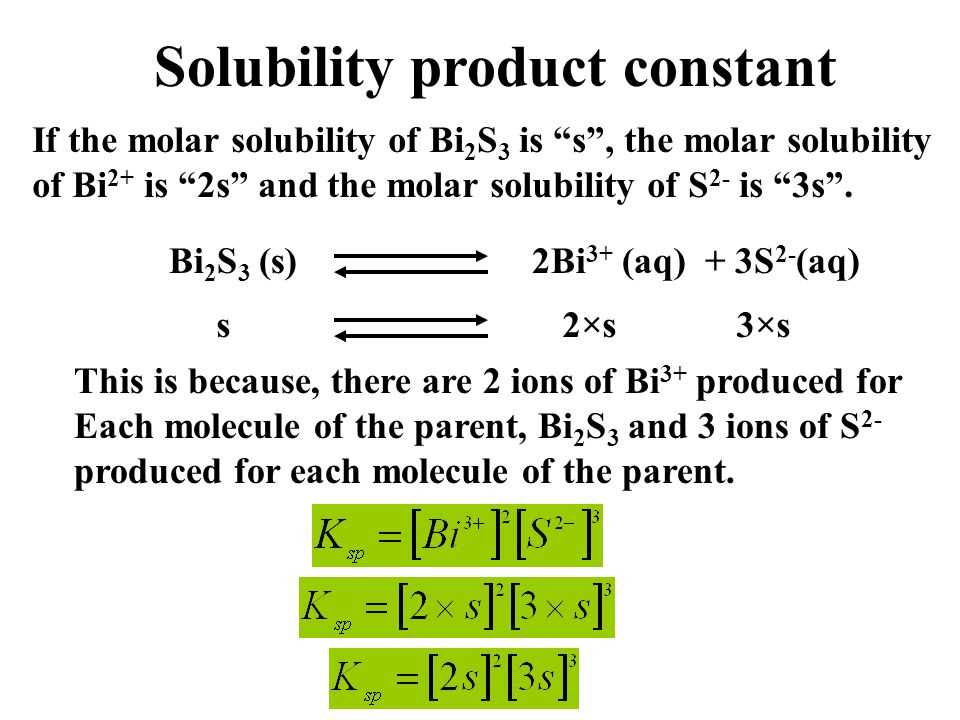
The solubility product constant (Ksp) is a measure of the solubility of a compound in a solvent. It is defined as the product of the concentrations of the ions in a saturated solution of the compound. Ksp is a thermodynamic equilibrium constant and is specific to each compound.
The solubility product constant is calculated by multiplying the concentrations of the ions in a saturated solution raised to the power of their respective stoichiometric coefficients in the balanced equation. For example, if the balanced equation for the dissolution of a compound is A(aq) + B(aq) ⇌ AB(s), the Ksp can be expressed as [A][B], where [A] and [B] are the concentrations of the ions A and B, respectively.
The value of Ksp indicates the extent of solubility of a compound. If the Ksp value is high, it means that the compound is highly soluble in the solvent. On the other hand, if the Ksp value is low, it indicates that the compound has low solubility in the solvent. The Ksp value does not depend on the amount of compound present in the solution, but rather on the concentrations of the ions.
The solubility product constant is important in determining the solubility of a compound and can be used to predict the formation of a precipitate when two solutions are mixed. If the product of the concentrations of the ions in the mixed solutions exceeds the Ksp value, a precipitate will form. This phenomenon is known as the common ion effect.
In summary, the solubility product constant (Ksp) is a measure of the solubility of a compound in a solvent. It is calculated by multiplying the concentrations of the ions in a saturated solution and is specific to each compound. The value of Ksp indicates the extent of solubility and can be used to predict the formation of a precipitate.
Examples of the common ion effect on solubility
The common ion effect is a phenomenon observed when the solubility of a given compound is reduced due to the presence of an ion that is already present in the solution. This effect can be seen in various chemical reactions and has important implications in everyday life.
One example of the common ion effect on solubility is the dissolution of calcium fluoride in water. Calcium fluoride is sparingly soluble in water, meaning that only a small amount of it will dissolve. However, when calcium fluoride is dissolved in a solution that already contains fluoride ions, the solubility of calcium fluoride is further reduced. This is because the fluoride ions from the solution compete with the fluoride ions from calcium fluoride to form calcium fluoride crystals, making it harder for calcium fluoride to dissolve.
Another example of the common ion effect is the solubility of silver chloride in water. Silver chloride is also sparingly soluble in water, but when it is dissolved in a solution that already contains chloride ions, the solubility of silver chloride decreases even further. This is due to the common ion effect, where the presence of chloride ions from the solution reduces the dissolution of silver chloride by forming insoluble silver chloride crystals.
- In summary, the common ion effect on solubility i