
Understanding how to count atoms and write chemical equations is a fundamental skill in chemistry. This worksheet provides practice problems to help students develop their proficiency in these areas. By working through the answer key, students can check their understanding and improve their knowledge of chemical formulas, balancing equations, and stoichiometry.
The answer key for this worksheet contains detailed explanations and step-by-step solutions. It includes examples of how to count the number of atoms in a molecule by using subscripts and coefficients. This is essential for correctly writing chemical formulas and balancing equations, as it determines the ratio of atoms involved in a reaction.
In addition to counting atoms, the answer key helps students learn how to balance chemical equations. Balancing equations ensures that the number of atoms on both sides of the equation is equal, as atoms cannot be created or destroyed in a chemical reaction. The key provides strategies and techniques for balancing equations and offers practice problems to reinforce this skill.
Furthermore, the answer key covers stoichiometry, which involves using the balanced chemical equations to determine the quantities of reactants and products in a reaction. It guides students through the process of converting between moles and grams, and provides practice problems to reinforce these calculations.
Overall, the counting atoms and writing chemical equations worksheet answer key is a valuable resource for chemistry students seeking to strengthen their understanding and skills in these foundational areas. By working through the key, students can gain confidence in their ability to manipulate chemical formulas, balance equations, and perform stoichiometric calculations.
Understanding Atoms and Their Symbols
Atoms are the building blocks of all matter. They are incredibly small particles that make up everything we see and touch. Each element on the periodic table has its own unique atom, characterized by its symbol. Understanding these symbols is essential in chemistry as they represent the identity and properties of each element.
The symbols of atoms are derived from their names, often using the first letter or two. For example, the symbol for oxygen is “O,” while the symbol for carbon is “C.” Some symbols have been derived from their Latin names, such as potassium (K) from “kalium.” Learning these symbols is important because they allow us to write chemical equations and communicate chemical reactions.
In addition to their symbols, atoms also have atomic numbers, which represent the number of protons in their nucleus. The number of protons determines the element’s identity. For example, carbon always has 6 protons, while oxygen always has 8 protons. Atomic numbers are displayed as a subscript to the left of the symbol. For example, the symbol for carbon is written as “C6,” indicating its atomic number.
Understanding atoms and their symbols is crucial in various scientific disciplines, including chemistry, physics, and biology. By knowing the symbols, scientists can easily identify elements and understand their behavior in chemical reactions. Additionally, symbols allow scientists to represent compounds and molecules, which are formed by the combination of atoms. Overall, atoms and their symbols are the foundation of our understanding of the physical world and play a significant role in advancing our scientific knowledge.
What Are Atoms?
An atom is the basic unit of matter. It is the smallest particle of an element that retains the chemical properties of that element. Atoms are incredibly small and cannot be seen with the naked eye. They are composed of even smaller particles called protons, neutrons, and electrons.
Protons are positively charged particles found in the nucleus of an atom. They have a relative mass of 1 and a relative charge of +1. Each element has a specific number of protons, known as its atomic number, which determines its identity. For example, all hydrogen atoms have 1 proton, while all oxygen atoms have 8 protons.
Neutrons are neutral particles found in the nucleus of an atom. They have a relative mass of 1 and no charge. The number of neutrons in an atom can vary, resulting in different isotopes of an element. Isotopes are atoms of the same element that have different numbers of neutrons.
Electrons are negatively charged particles that exist in different energy levels, or shells, around the nucleus of an atom. They have a negligible mass and a relative charge of -1. The outermost shell, known as the valence shell, determines the atom’s chemical behavior and its ability to bond with other atoms.
In summary, atoms are the building blocks of matter. They are composed of protons, neutrons, and electrons. The arrangement and combination of these particles determine the properties and behavior of different elements.
The Symbolism of Atoms

Atoms are the fundamental building blocks of matter, and understanding their symbolism is essential in the field of chemistry. Each atom is represented by its chemical symbol, which is a shorthand way of identifying the element it belongs to. The symbols are derived from the element’s name or its Latin name, and they provide a concise and universally understood way of referring to specific atoms.
The symbolism of atoms goes beyond their chemical symbols. Atoms are also associated with certain characteristics and qualities that are represented symbolically. For example, hydrogen, the lightest element, is often used as a symbol of purity and simplicity. It is also associated with the concept of beginnings, as it is the primary building block of stars and the universe itself.
In addition to their symbolic representations, atoms are also used in various symbolic systems in different cultures. For example, in ancient alchemy, different elements and their corresponding atoms were believed to have different properties and powers. These symbolic associations often extended to the spiritual and metaphysical realm, with each atom representing a different aspect of the natural world and the human experience.
Overall, the symbolism of atoms plays a crucial role in the understanding and communication of chemical concepts. By using symbols to represent atoms, scientists can convey complex ideas in a concise and standardized manner. Additionally, the symbolic associations of different elements and their atoms add depth and meaning to our understanding of the natural world and our place within it.
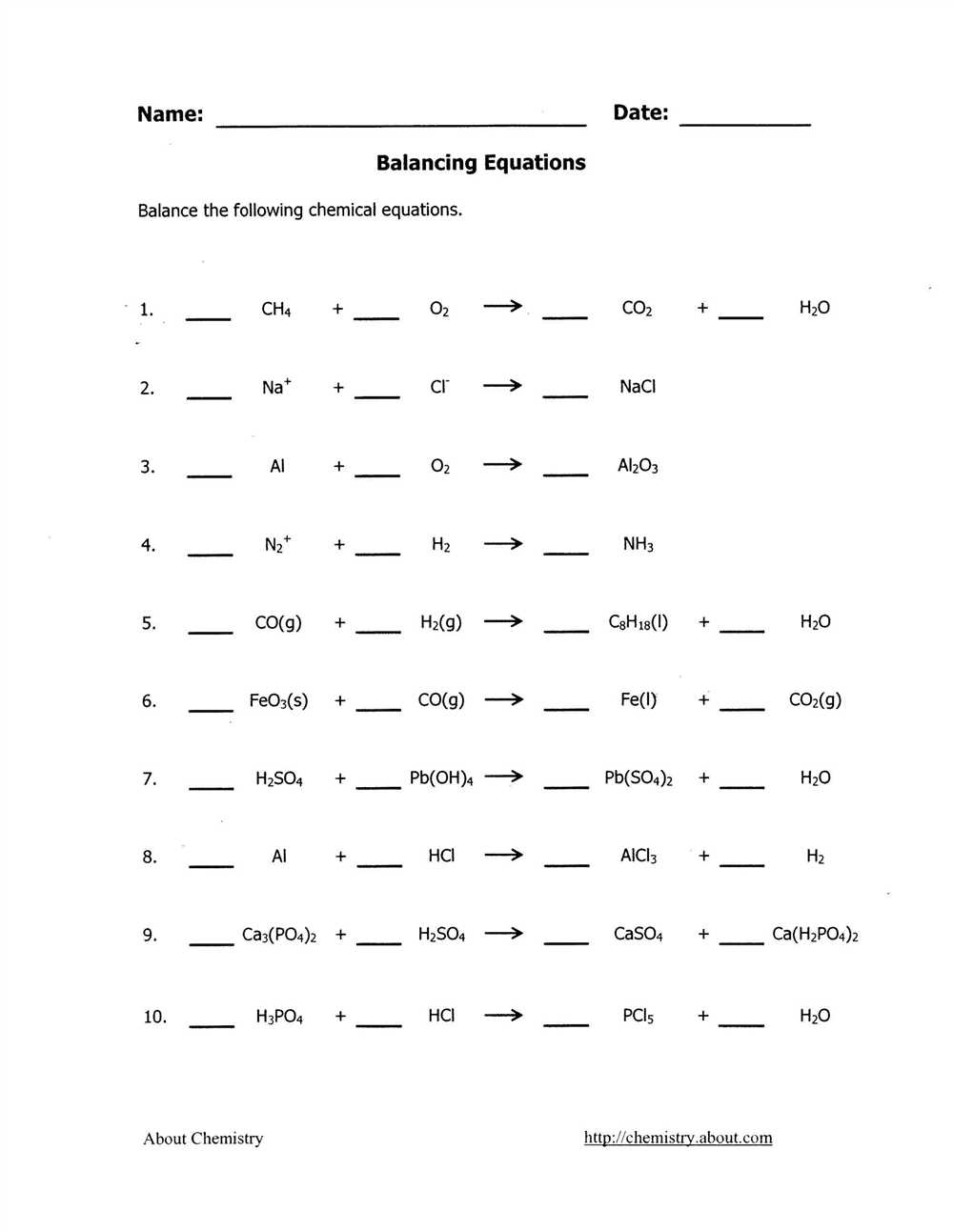
Balancing Chemical Equations
When it comes to studying chemical reactions, one important skill is the ability to balance chemical equations. A chemical equation is a representation of a chemical reaction using chemical symbols and formulas. It shows the reactants on the left side and the products on the right side, separated by an arrow.
To balance a chemical equation means to make sure that there is an equal number of each type of atom on both sides of the equation. This is important because atoms can neither be created nor destroyed during a chemical reaction. The principle of conservation of mass states that the total mass of the reactants must be equal to the total mass of the products.
The process of balancing chemical equations involves adjusting the coefficients in front of the chemical formulas in the equation. Coefficients are used to indicate the number of molecules or atoms of each substance participating in the reaction. By changing the coefficients, we can ensure that the number of atoms on both sides of the equation is the same.
To begin balancing an equation, it is helpful to start with the most complex molecule or compound and then work your way down to simpler ones. One strategy is to balance the atoms that appear in the fewest compounds first. This helps in maintaining the balance while adjusting the coefficients of other compounds.
It is important to note that in balancing chemical equations, only coefficients can be changed, not subscripts. Changing subscripts would mean altering the chemical identity of the substance, which is not allowed in a balanced equation.
Overall, balancing chemical equations is a fundamental skill in chemistry. It allows us to represent chemical reactions accurately and understand the stoichiometry of reactions. By following specific strategies and rules, we can ensure that the number of atoms is conserved and that the equation represents a balanced chemical reaction.
Importance of Balancing Chemical Equations
The process of balancing chemical equations is crucial in understanding and predicting chemical reactions. It allows scientists to determine the exact ratios in which reactant molecules combine to form products, and to ensure that the law of conservation of mass is upheld. Balancing chemical equations provides a quantitative representation of chemical reactions, allowing scientists to make accurate calculations and predictions.
One of the primary reasons balancing chemical equations is important is that it ensures that all atoms are accounted for in a reaction. By balancing the coefficients of reactants and products, the same number and type of atoms are present on both sides of the equation. This is essential because atoms cannot be created or destroyed in a chemical reaction, according to the law of conservation of mass. Balancing the equation allows scientists to confirm that no atoms are lost or gained during a reaction.
Furthermore, balancing chemical equations provides essential information about the stoichiometry of a reaction, which is the relationship between the quantities of reactants and products. The balanced equation shows the ratio in which reactant molecules combine and the ratio of product molecules that are formed. This is crucial for determining how much of each substance is required in a reaction and how much product will be obtained. Without balancing the equation, accurate calculations and predictions about the reaction are not possible.
In conclusion, balancing chemical equations is fundamental in chemistry as it ensures the conservation of mass, accounting for all atoms involved in a reaction. It also provides valuable information about the stoichiometry of a reaction, allowing scientists to make accurate calculations and predictions. Balancing chemical equations is an essential skill in understanding and studying chemical reactions.
Steps to Balance a Chemical Equation
In order to balance a chemical equation, you need to make sure that the number of atoms on both sides of the equation are equal. This involves following a few simple steps:
- Write down the unbalanced equation: Start by writing down the chemical equation with the reactants on the left side of the arrow and the products on the right side.
- Count the number of atoms on each side: Count the number of atoms for each element on both sides of the equation. Keep track of these numbers.
- Balance the equation starting with the most complex molecule: Begin by balancing the atoms in the most complex molecule. This is typically the molecule with the most atoms or the one that appears only once on each side of the equation.
- Adjust the coefficients: Once you have balanced the atoms in the most complex molecule, adjust the coefficients of the other molecules to ensure that the number of atoms on each side of the equation are equal.
- Check the equation: After adjusting the coefficients, double-check that the number of atoms for each element are equal on both sides of the equation.
By following these steps, you can ensure that your chemical equation is balanced and accurately represents the chemical reaction that is occurring.
Writing Chemical Formulas and Equations
Writing chemical formulas and equations is an essential skill in chemistry. It allows us to communicate the composition and reactions of substances in a concise and standardized way. Chemical formulas provide a way to represent the elements and their ratios in a compound, while chemical equations show the reactants and products involved in a chemical reaction.
Chemical Formulas: A chemical formula represents the types and numbers of atoms present in a compound. It consists of element symbols and subscripts, indicating the number of each element in the compound. For example, the formula for water is H2O, which indicates that there are two hydrogen (H) atoms and one oxygen (O) atom in each molecule of water.
Chemical Equations: A chemical equation represents a chemical reaction using chemical formulas and other symbols. It shows the reactants on the left side and the products on the right side, separated by an arrow. Coefficients are often used to balance the equation, ensuring that the number of atoms on both sides is equal. For example, the equation for the reaction between hydrogen gas (H2) and oxygen gas (O2) to form water is 2H2 + O2 → 2H2O.
When writing chemical formulas and equations, it is important to follow certain rules. These include using proper symbols for elements and compounds, balancing the equation to preserve the law of conservation of mass, and indicating the state of matter for each substance (solid, liquid, gas, or aqueous solution).
In summary, writing chemical formulas and equations is a fundamental skill in chemistry that allows scientists to accurately represent the composition and reactions of substances. It involves using chemical symbols and subscripts to represent atoms and compounds, and balancing equations to ensure the conservation of mass. By mastering this skill, chemists can effectively communicate their findings and contribute to the understanding of the scientific world.
Basics of Chemical Formulas
In chemistry, chemical formulas are used to represent the composition of substances. A chemical formula is a combination of symbols that represent the elements present in a compound and the number of atoms of each element. The symbols used are derived from the names of the elements and are unique to each element. For example, the symbol “H” represents hydrogen, “O” represents oxygen, and “Na” represents sodium.
The subscripts in a chemical formula indicate the number of atoms of each element in a compound. For example, in the formula H2O, there are two atoms of hydrogen and one atom of oxygen. These subscripts are used to balance chemical equations and to determine the molar ratios between different elements in a compound.
Chemical formulas follow certain rules and conventions. The first element in a compound is usually written without a subscript, while the subscripts for subsequent elements are written immediately after the symbol. If there is only one atom of a particular element, the subscript “1” is usually omitted. Additionally, parentheses are used when a group of atoms is repeated in the compound, and the subscript outside the parentheses indicates the number of repetitions.
Chemical formulas are essential for understanding and communicating the composition of substances in chemistry. They provide a concise and standardized way of representing compounds and their constituents. By understanding the basics of chemical formulas, one can analyze and interpret chemical reactions, predict chemical properties, and study the behavior of substances in various reactions and conditions.