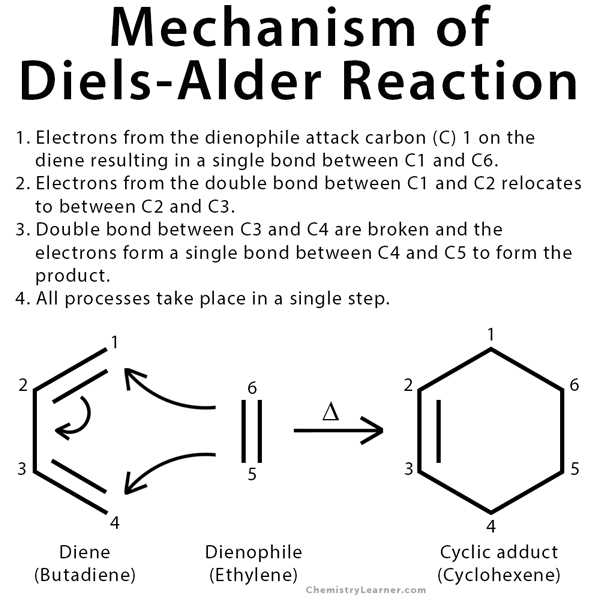
The Diels Alder reaction is a powerful tool in organic chemistry, allowing for the synthesis of complex cyclic compounds. It involves the reaction of a conjugated diene with a dienophile to form a cyclohexene ring. This reaction is known for its high regio- and stereo-selectivity, making it a valuable tool for organic chemists.
Understanding and mastering the Diels Alder reaction requires practice, and one way to gain proficiency is through solving practice problems. In this article, we will provide a set of Diels Alder practice problems along with detailed answers, allowing students and practitioners to test their knowledge and improve their understanding of this important reaction.
These practice problems will cover various aspects of the Diels Alder reaction, including the identification of the diene and dienophile, predicting the regio- and stereochemistry of the product, and determining the reaction conditions. By solving these problems, readers will not only gain a deeper understanding of the Diels Alder reaction but also strengthen their problem-solving skills in organic chemistry.
Diels-Alder Practice: Understanding the Reaction Mechanism
In the study of organic chemistry, the Diels-Alder reaction is an essential topic to understand. This reaction involves the formation of a cyclic compound through the combination of a diene and a dienophile. To fully grasp this reaction, it is crucial to understand the underlying mechanism.
The Diels-Alder reaction follows a concerted mechanism, meaning that all bond-breaking and bond-forming steps occur in a single step without any intermediates. The diene and dienophile undergo a [4+2] cycloaddition, where four π-electrons from the diene and two π-electrons from the dienophile come together to form six new σ-bonds.
The reaction begins with the approach of the diene and dienophile, resulting in the formation of a transition state. This transition state is characterized by a cyclic arrangement of the six atoms involved in the reaction. The diene undergoes a conformational change, bending to bring its two π-bonds into a favorable position for bonding with the dienophile.
The next step involves the interaction of the diene and dienophile orbitals. The highest occupied molecular orbital (HOMO) of the diene, which contains the π-electrons, interacts with the lowest unoccupied molecular orbital (LUMO) of the dienophile. This interaction leads to the formation of new σ-bonds and the breaking of existing π-bonds.
Overall, understanding the Diels-Alder reaction mechanism is crucial for predicting the outcome of reactions and designing novel synthetic routes. By analyzing the orbitals involved and the conformational changes of the diene, chemists can gain insight into the regioselectivity and stereoselectivity of the reaction.
Key Concepts and Definitions
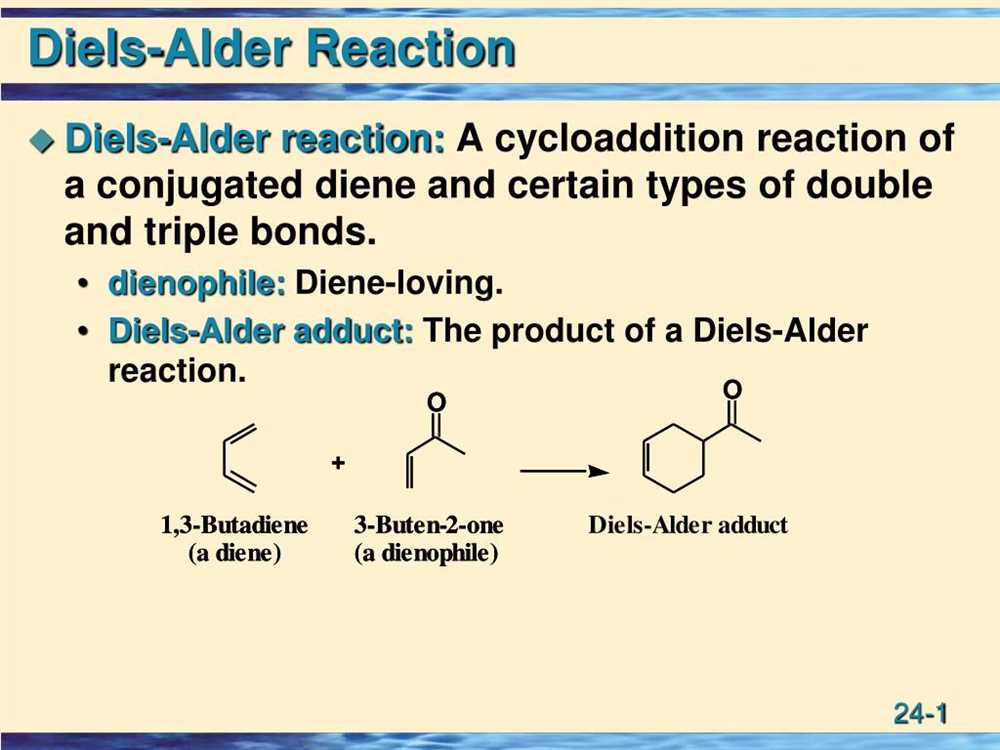
Diels-Alder reaction: The Diels-Alder reaction is a pericyclic reaction between a conjugated diene and a dienophile to form a cyclohexene ring. It is a concerted reaction that proceeds through a cyclic transition state.
Conjugated diene: A conjugated diene is a hydrocarbon molecule that contains multiple carbon-carbon double bonds separated by a single bond. The double bonds are typically separated by one or two carbon atoms.
- Dienophile: A dienophile is a molecule that reacts with a diene in a Diels-Alder reaction. It is typically an electron-deficient species, such as a double or triple bond.
- Endo selectivity: Endo selectivity refers to the preference of the Diels-Alder reaction to form the endo product, where the substituent on the dienophile is positioned inside the newly formed cyclohexene ring.
- Exo selectivity: Exo selectivity refers to the preference of the Diels-Alder reaction to form the exo product, where the substituent on the dienophile is positioned outside the newly formed cyclohexene ring.
- Stereochemistry: Stereochemistry refers to the three-dimensional arrangement of atoms in a molecule. In the Diels-Alder reaction, stereochemistry can play a role in determining the regioselectivity and stereoselectivity of the reaction.
The key concepts and definitions presented above are essential for understanding and predicting the outcome of Diels-Alder reactions. These reactions are widely used in organic synthesis for the construction of cyclic structures and the formation of carbon-carbon bonds. Understanding the role of the diene, dienophile, and stereochemistry is crucial for achieving the desired selectivity in these reactions. Additionally, the ability to predict whether the reaction will proceed via endo or exo selectivity is important for designing synthetic routes and controlling the outcome of the reaction.
Reaction Conditions and Reactivity
Diels-Alder reactions are a type of cycloaddition reaction that involve the formation of a new cyclic compound from a diene and a dienophile. The reactivity of the diene and dienophile can be influenced by several factors, including the reaction conditions and the nature of the reactants.
The reaction conditions, such as temperature, solvent, and catalyst, can have a significant impact on the rate and selectivity of the Diels-Alder reaction. For example, increasing the temperature can accelerate the reaction by providing the necessary energy for the formation of the new bonds. Solvents can also affect the reaction by stabilizing or destabilizing the reactants or intermediates. Additionally, the use of catalysts can enhance the reactivity of the diene or dienophile, leading to faster and more selective reactions.
The reactivity of the diene and dienophile is determined by their electronic and steric properties. Electron-withdrawing groups on the dienophile can increase its reactivity by polarizing the diene and facilitating the formation of the new bonds. On the other hand, electron-donating groups on the diene can increase its reactivity by stabilizing the transition state. Steric hindrance can also influence the reactivity by preventing or facilitating the approach of the reactants or the formation of the new bonds. Therefore, the choice of diene and dienophile, as well as their substituents, can greatly affect the outcome of the Diels-Alder reaction.
- Reaction conditions, such as temperature, solvent, and catalyst, play a crucial role in controlling the rate and selectivity of the Diels-Alder reaction.
- Electronic and steric properties of the diene and dienophile determine their reactivity in the Diels-Alder reaction.
Stereochemistry in Diels-Alder Reactions
The Diels-Alder reaction is a powerful synthetic tool that allows the formation of complex cyclic compounds with high stereoselectivity. This reaction involves the addition of a diene and a dienophile, resulting in the formation of a new carbon-carbon bond and the generation of a cyclohexene ring. The stereochemistry of the products obtained in Diels-Alder reactions is highly dependent on the orientation of the diene and dienophile and the stereochemistry of the transition state.
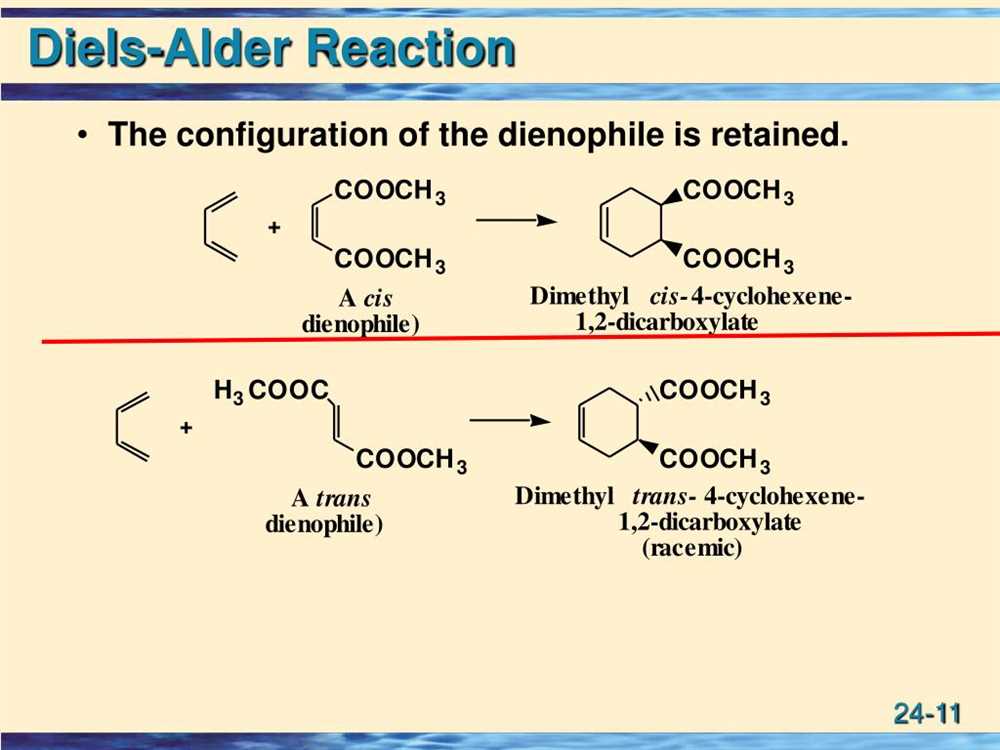
In a stereospecific Diels-Alder reaction, the stereochemistry of the products is dictated by the stereochemistry of the reactants. For example, if a cis-1,3-diene reacts with a trans-dienophile, the resulting product will maintain the stereochemistry of the reactants. This means that if the diene is cis, the product will also be cis, and if the diene is trans, the product will also be trans. This stereochemical outcome is a result of the concerted mechanism of the Diels-Alder reaction, which prevents the formation of intermediate stereoisomers.
In a stereoselective Diels-Alder reaction, the stereochemistry of the products is influenced by the stereochemistry of the reactants, but multiple stereochemical outcomes are possible. For example, if a cis-1,3-diene reacts with a cis-dienophile, two possible products can be formed: one with cis stereochemistry and one with trans stereochemistry. The favored product will generally be determined by factors such as steric hindrance and electronic effects. The stereochemistry of the dienophile can also play a role in determining the stereochemistry of the products.
Overall, the stereochemistry in Diels-Alder reactions is a complex interplay of the stereochemistry of the reactants, the stereochemistry of the transition state, and various influencing factors. Understanding the stereochemical outcomes in these reactions is crucial for synthetic chemists in designing efficient synthetic routes to complex cyclic compounds.
Diels-Alder Practice Questions: Reactants and Products
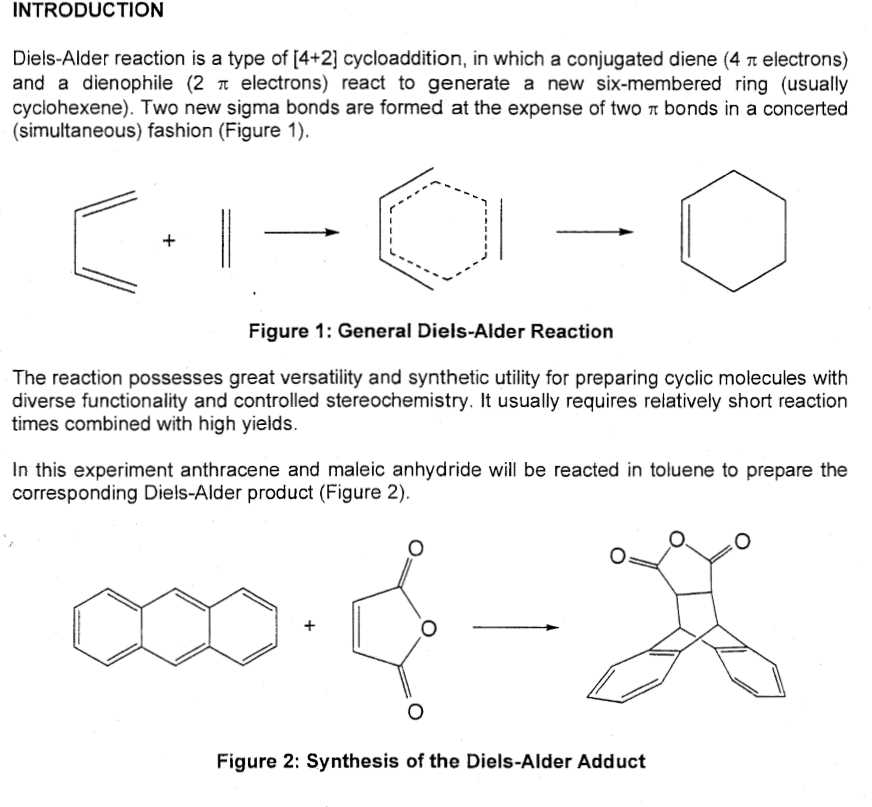
The Diels-Alder reaction is a powerful tool in organic synthesis that allows for the formation of new carbon-carbon bonds. This reaction involves the cycloaddition of a diene and a dienophile to produce a cyclohexene ring system. In order to successfully predict the products of a Diels-Alder reaction, it is important to consider the reactants and their reactivity.
1. What are the typical reactants in a Diels-Alder reaction?
In a Diels-Alder reaction, the typical reactants are a diene and a dienophile. The diene is a molecule containing two double bonds, while the dienophile is a molecule containing one or more double bonds that can react with the diene. The diene and dienophile usually contain different functional groups, which contribute to the overall reactivity of the reaction.
2. How do the substituents on the diene and dienophile affect the reaction?
The substituents on the diene and dienophile can have a significant impact on the reaction. Electron-withdrawing groups on the dienophile can make it more reactive, while electron-donating groups can decrease its reactivity. Similarly, electron-withdrawing groups on the diene can increase its reactivity, while electron-donating groups can decrease its reactivity. These substituents can also influence the regiochemistry and stereoselectivity of the reaction, leading to different products.
3. What are some examples of reactants and products in a Diels-Alder reaction?
An example of a diene could be 1,3-butadiene, which contains two conjugated double bonds. A dienophile could be maleic anhydride, which contains a double bond and an anhydride functional group. When these two reactants undergo a Diels-Alder reaction, they can produce endo-3-cyclohexene carboxylic acid. The specific product formed will depend on the regiochemistry and stereochemistry of the reaction.
In another example, a diene such as cyclopentadiene could react with a dienophile like methyl acrylate. This reaction could yield exo-4-methylene-7-oxobicyclo[2.2.1]hept-5-ene as the product.
In summary, the reactants and their substituents play a crucial role in determining the products of a Diels-Alder reaction. The reactivity and electronic properties of the diene and dienophile, as well as the regiochemistry and stereochemistry of the reaction, must be considered in order to predict the outcome of the reaction.
Recognizing Diene and Dienophile
The Diels-Alder reaction is a powerful tool in organic chemistry for the synthesis of cyclic compounds. In this reaction, a diene and a dienophile come together to form a cycloadduct. The diene is a molecule that contains two double bonds, while the dienophile is a molecule that contains a double bond and an electron-withdrawing group.
By recognizing the diene and dienophile in a given reaction, chemists can determine the direction of the reaction and predict the outcome. The diene is typically the electron-rich component, while the dienophile is the electron-poor component. This electron density difference drives the reaction and determines which bond will form the new cyclic compound.
Identifying the diene and dienophile can be done by analyzing the structure and the nature of the substituents. The diene is often a conjugated system with a long hydrocarbon chain, while the dienophile can contain various functional groups such as carbonyl, nitro, or cyano groups. Additionally, the dienophile should have an electron-withdrawing group attached to the double bond.
A common example of a diene is 1,3-butadiene, which has a conjugated system of four carbon atoms and two double bonds. An example of a dienophile is maleic anhydride, which contains a double bond and two carbonyl groups as electron-withdrawing groups.
Predicting the Major Products
Predicting the major products in Diels-Alder reactions can be a challenging task, but by understanding the key factors that contribute to product formation, it becomes easier to make accurate predictions. One important factor to consider is the reactivity of the diene and the dienophile. The diene should have a relatively low LUMO energy level, while the dienophile should have a high HOMO energy level.
Additionally, the substitution pattern of the diene and the dienophile can also influence the regioselectivity of the reaction. For example, if the diene contains electron-withdrawing groups, the reaction is more likely to occur at the site of least substitution. On the other hand, if the dienophile contains electron-withdrawing groups, the reaction is more likely to occur at the site of greatest substitution.
In some cases, steric factors can also play a role in determining the major product. Hindered dienes or dienophiles may preferentially react at less sterically hindered positions. This can be influenced by the size and orientation of substituents on the diene and dienophile.
Overall, predicting the major products in Diels-Alder reactions requires a thorough understanding of the reactivity and substitution patterns of the reactants, as well as consideration of steric factors. By considering these factors, chemists can make more accurate predictions to guide their experimental work.
Key phrases: predicting the major products, Diels-Alder reactions, reactivity, diene, dienophile, substitution pattern, regioselectivity, electron-withdrawing groups, steric factors
Evaluating the Stereoselectivity of the Reaction
Stereoselectivity is an important concept in organic chemistry that refers to the preferential formation of one stereoisomer over another in a chemical reaction. In the case of Diels-Alder reactions, stereoselectivity can be evaluated by examining the arrangement of substituents on the reacting diene and dienophile, as well as the geometry of the resulting cycloadduct.
When evaluating stereoselectivity, it is important to consider the regioselectivity of the Diels-Alder reaction, which determines the orientation of the new bonds formed during the cycloaddition. The reaction can occur either endo-selectively or exo-selectively, depending on the relative orientation of the diene and dienophile. Endo-selective reactions result in the formation of a five-membered ring with the substituents on the diene pointing towards the dienophile, while exo-selective reactions form a six-membered ring with the substituents pointing away from the dienophile.
In addition to regioselectivity, stereoselectivity also depends on the configuration of the starting materials. For example, if the diene and dienophile have fixed stereochemistry, the stereochemistry of the resulting cycloadduct will also be fixed. However, if one or both of the starting materials have flexible stereochemistry, there may be multiple stereoisomers formed in the reaction, with different spatial arrangements of the substituents on the cycloadduct.
To evaluate the stereoselectivity of a Diels-Alder reaction, one can analyze the product using spectroscopic techniques such as nuclear magnetic resonance (NMR) and mass spectrometry (MS) to determine the relative abundance of different stereoisomers. Additionally, computational methods such as molecular modeling can be used to predict the stereochemistry of the cycloadduct based on the starting materials and reaction conditions.
In conclusion, evaluating the stereoselectivity of a Diels-Alder reaction involves considering the regioselectivity of the reaction, the configuration of the starting materials, and analyzing the resulting cycloadduct using spectroscopic and computational methods. Understanding the factors that influence stereoselectivity is essential for designing and optimizing synthetic routes in organic chemistry.