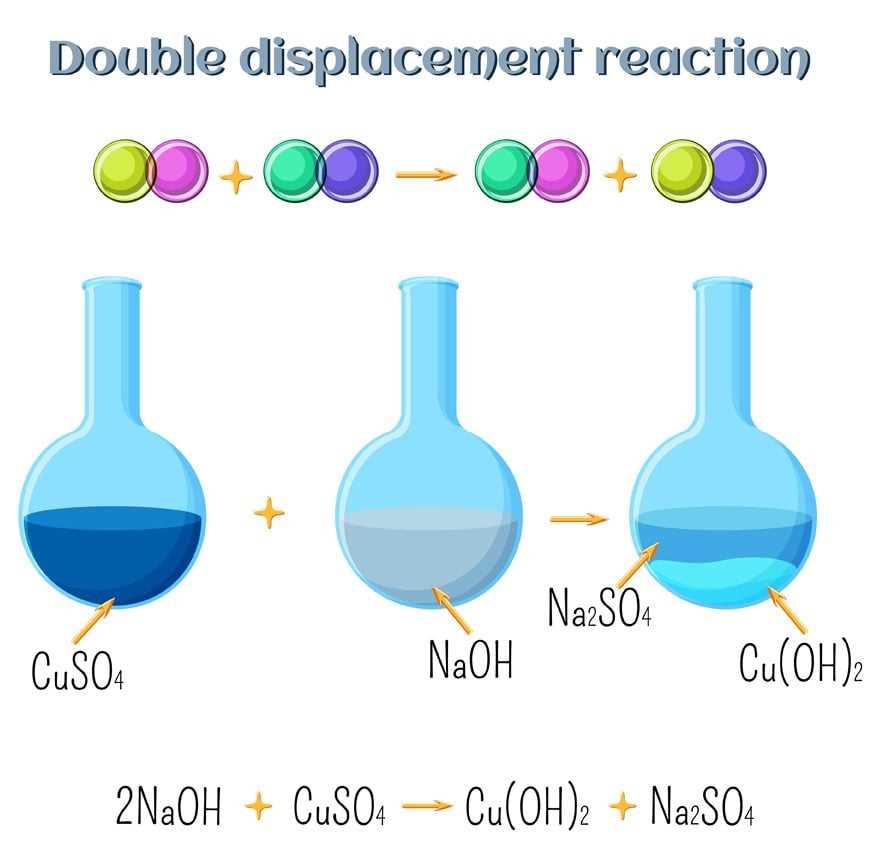
In a chemistry lab, students often perform experiments to study various chemical reactions and their outcomes. One common type of reaction is a double replacement reaction, also known as a metathesis reaction. These reactions involve the exchange of ions between two compounds, leading to the formation of new products.
During a double replacement reaction, cations and anions from two compounds switch places, resulting in the formation of two new compounds. This type of reaction can be used to determine the solubility of various compounds in water. By observing whether a precipitate forms or if the reaction remains soluble, students can gain insight into the solubility rules and predict the outcome of similar reactions.
The solubility of a compound refers to its ability to dissolve in a solvent, usually water. Some compounds are highly soluble and readily dissolve in water, while others are insoluble and form precipitates. Solubility rules can help determine the solubility of different compounds in water based on the types of ions involved. These rules provide a basic understanding of double replacement reactions and can be used to predict the products and solubility of compounds.
Double Replacement Reactions and Solubility Lab Answers
Double replacement reactions and solubility lab answers are important in chemistry as they help determine the products that form when two aqueous solutions react. These reactions involve the exchange of positive ions between compounds, resulting in new compounds and potentially precipitates. The solubility of compounds plays a crucial role in determining whether a reaction will occur and which products will form.
In the lab, a common way to determine solubility and predict double replacement reactions is by using a solubility table. This table lists the solubility of various compounds in water and provides insight into which compounds will dissolve or form a precipitate. For example, if a reaction between two solutions results in the formation of a compound that is insoluble according to the solubility table, a precipitate will likely form.
Additionally, conducting experiments and analyzing the resulting precipitates can provide further information about double replacement reactions and solubility. By observing the color, texture, and chemical properties of the precipitate, scientists can make deductions about the nature of the compounds involved and validate the predictions made based on the solubility table.
Understanding double replacement reactions and solubility is essential for various applications in chemistry, including the synthesis of new compounds, the analysis of unknown substances, and the design of chemical reactions. By studying the outcomes of double replacement reactions and analyzing the solubility of compounds, scientists can gain valuable insights into the behavior of different substances and make informed decisions in chemical research and industry.
What are double replacement reactions?
Double replacement reactions, also known as double displacement reactions or metathesis reactions, are chemical reactions that involve the exchange of ions between two compounds. In these reactions, the positive and negative ions of two compounds switch places to form two new compounds.
In a typical double replacement reaction, the cations (positively charged ions) of the two compounds swap places, while the anions (negatively charged ions) also exchange partners. The reactants and products in double replacement reactions are often soluble ionic compounds.
A key requirement for double replacement reactions to occur is the formation of a precipitate, which is an insoluble compound that is formed when two aqueous solutions react. The formation of a precipitate indicates that a chemical change has occurred.
Double replacement reactions often result in the formation of a new solid, a gas, or a precipitate. They are commonly used in laboratory experiments to determine the solubility of different compounds and to identify the products of a chemical reaction.
The general equation for a double replacement reaction can be written as: AB + CD → AD + CB, where A, B, C, and D represent different elements or compounds.
Overall, double replacement reactions play an important role in understanding the behavior of different compounds in solution and are widely studied in chemistry.
Understanding solubility in double replacement reactions
Solubility refers to the ability of a substance, known as the solute, to dissolve in another substance, known as the solvent. In double replacement reactions, two compounds react to form two new compounds, with the positive ions switching places. Understanding the solubility of these compounds is crucial in predicting the outcome of these reactions.
The solubility of a compound depends on several factors, including the nature of the solvent and the types of ions present in the compound. Some compounds are highly soluble in water, while others are completely insoluble. Solubility rules can be used as a guide to predict the solubility of different compounds.
The solubility rules for double replacement reactions are as follows:
- Most nitrates (NO₃-) are soluble.
- Most compounds containing alkali metal ions (Li+, Na+, K+, etc.) and ammonium ions (NH₄+) are soluble.
- Most chlorides (Cl-), bromides (Br-), and iodides (I-) are soluble, with the exception of those with silver ions (Ag+), lead ions (Pb2+), and mercury ions (Hg2²+).
- Most sulfates (SO₄²-) are soluble, with the exception of those with calcium ions (Ca²+), strontium ions (Sr²+), barium ions (Ba²+), lead ions (Pb2+), and silver ions (Ag+).
- Most carbonates (CO₃²-), phosphates (PO₄³-), sulfides (S²-), and hydroxides (OH-) are insoluble, with the exception of those with alkali metal ions (Li+, Na+, K+, etc.) and ammonium ions (NH₄+).
By following these solubility rules, it becomes easier to predict the products of double replacement reactions and determine whether a precipitate will form. This knowledge is essential in fields such as chemistry, environmental science, and pharmaceuticals, as it allows scientists to understand and control the reactions that occur in various processes.
Factors affecting solubility in double replacement reactions
Double replacement reactions occur when two compounds exchange ions to form two new compounds. In these reactions, the solubility of the compounds plays a crucial role in determining whether a reaction will occur and which products will form. There are several factors that can affect the solubility of compounds in double replacement reactions.
One important factor is the nature of the ions being exchanged. Some ions tend to form highly soluble compounds, while others form insoluble compounds. For example, compounds containing alkali metal ions (such as sodium or potassium) and nitrate (NO3-) or acetate (CH3COO-) ions are generally highly soluble in water. On the other hand, compounds containing ions like carbonate (CO3^2-) or sulfide (S^2-) tend to be insoluble.
The concentration of the ions in solution also affects solubility. According to Le Chatelier’s principle, increasing the concentration of one of the ions in solution can shift the equilibrium of the reaction, causing a decrease in solubility. For example, if the concentration of a chloride ion (Cl-) is increased in a reaction between a silver ion (Ag+) and a chloride ion (Cl-), the solubility of the resulting silver chloride (AgCl) will decrease.
The temperature of the solution can also impact solubility. In general, an increase in temperature increases the solubility of most compounds. This is because increasing temperature provides more kinetic energy to the particles, allowing them to overcome the attractive forces holding them together and dissolve more easily. However, there are exceptions to this rule, such as the solubility of some salts, which may decrease with increasing temperature.
In conclusion, the solubility of compounds in double replacement reactions is influenced by factors such as the nature of the ions being exchanged, the concentration of the ions in solution, and the temperature of the solution. Understanding these factors can help predict the outcome of double replacement reactions and explain the formation of specific products.
Experimental Setup for Double Replacement Reactions and Solubility Lab
In the double replacement reactions and solubility lab, the experimental setup involves several components to observe and analyze the reactions between different aqueous solutions. These components include various chemical reagents, test tubes, a hot plate, a thermometer, and a stirring rod.
To begin the lab, different aqueous solutions are prepared, each containing a specific chemical compound. These solutions are carefully labeled to avoid any confusion during the experiment. The solutions are typically prepared by dissolving the required amount of solid compound in distilled water.
Test Tube Setup
The next step involves setting up a series of test tubes for the reactions. Each test tube is labeled with the name of the respective reactant solution it will contain. The test tubes are usually placed in a test tube rack to keep them organized and stable during the experiment.
Reaction Procedure
One by one, the reactant solutions are added to the test tubes in a specific order. The order is usually determined by the solubility rules and the desired reaction. The solutions are added in small quantities using a dropper or pipette to ensure accurate measurement.
After each addition, the mixture is gently stirred using the stirring rod to ensure uniform mixing. The reaction can be observed visually, and any changes, such as color or the formation of a precipitate, are noted.
Temperature Control
During the experiment, the temperature of the reaction mixture is also monitored. This is done using a thermometer, which is immersed in the solution. The hot plate is used to heat the reaction mixture, if necessary, to promote the reaction or enhance the solubility of certain compounds.
Observations and Analysis
Throughout the experimental procedure, the observations and changes in the reaction mixture are noted. These observations are analyzed based on the solubility rules and known reactions between different compounds. The purpose is to identify any double replacement reactions and understand the solubility properties of the compounds being tested.
Finally, the lab results are summarized and analyzed to draw conclusions about the solubility of the compounds and the occurrence of double replacement reactions in different aqueous solutions.
Procedure for Conducting Double Replacement Reactions and Solubility Lab
In order to conduct a double replacement reaction and solubility lab, it is important to follow a step-by-step procedure to ensure accurate results. The following is a general outline of the procedure:
Gather Materials
Before starting the lab, gather all the necessary materials and equipment. This may include test tubes, test tube racks, a dropper, a pipette, a hot plate, chemicals (such as sodium chloride, silver nitrate, calcium chloride, and sodium carbonate), and safety equipment (such as gloves and goggles).
Prepare Test Tubes
Clean and label the test tubes to prevent contamination. It is important to have a separate test tube for each reaction you plan to perform.
Prepare Chemical Solutions
Measure out the required amounts of each chemical and dissolve them in water to create the necessary chemical solutions. Be sure to record the concentrations of each solution for future reference.
Perform Double Replacement Reactions
Add the appropriate amount of each chemical solution to the corresponding test tubes, following the reactions specified in the lab instructions. Observe any changes in color or formation of precipitates.
Record Observations
Record any observations or changes that occur during the reactions. This may include color changes, gas formation, or the appearance of a precipitate.
Determine Solubility
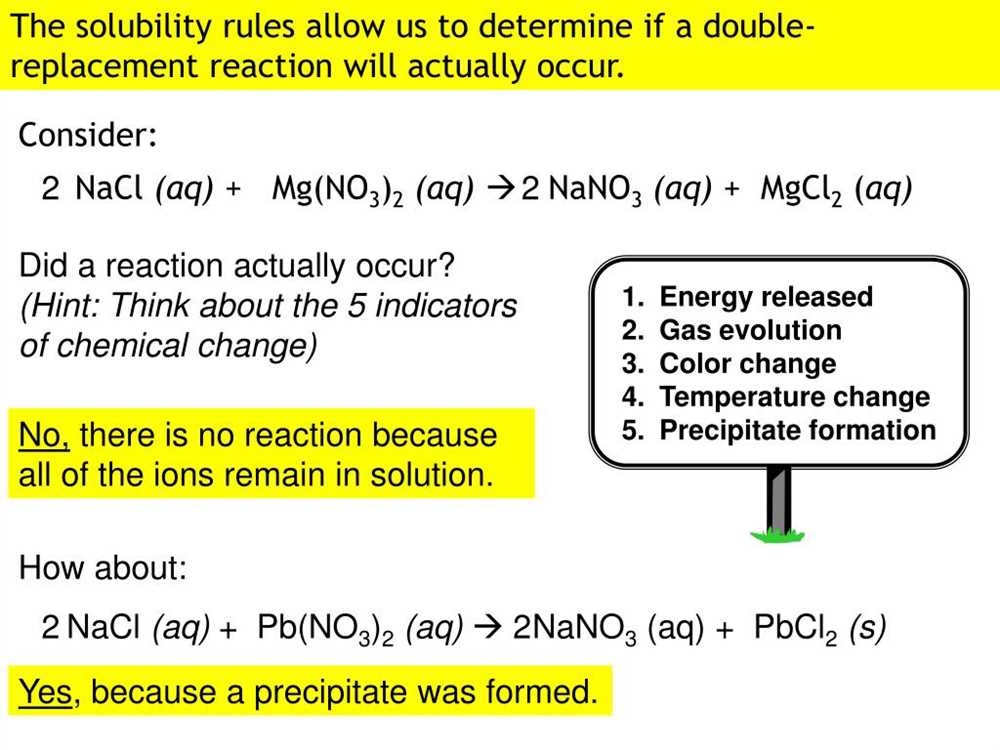
Using the observed results, determine the solubility of the products formed in each reaction. This can be done by comparing the observed precipitates to known solubility rules. Record the solubility of each product.
Analyze the Results
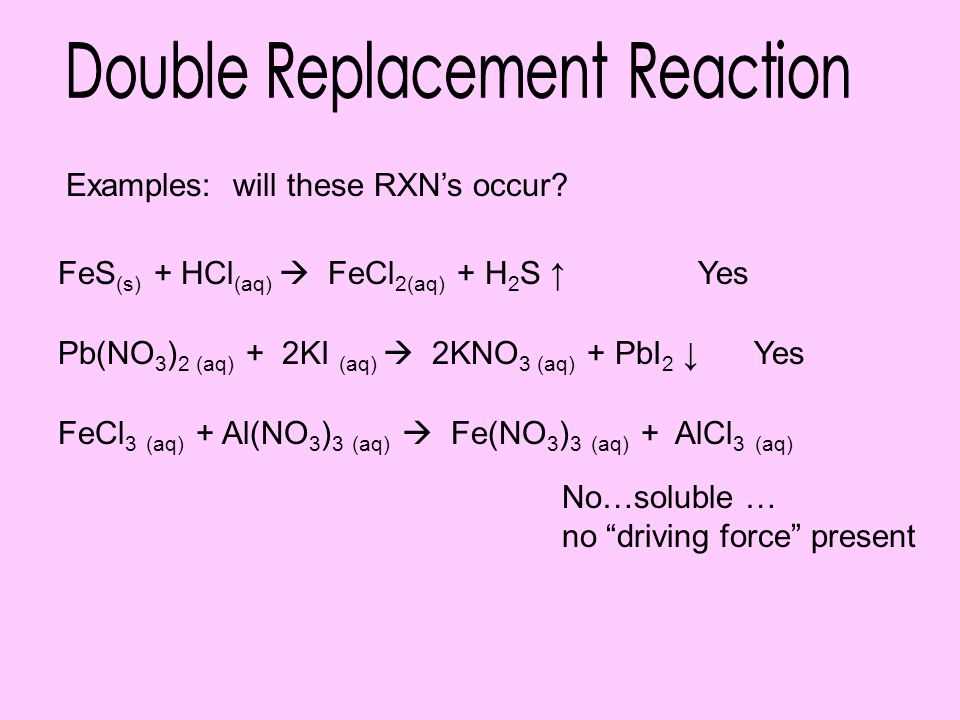
Analyze the results of the lab and draw conclusions based on the observations and solubility data. Identify any patterns or trends that may be present.
By following these steps, you can successfully conduct a double replacement reaction and solubility lab, allowing you to explore the relationship between different chemicals and their solubility in water.
Data Collection and Observations during the Lab
During the double replacement reactions and solubility lab, several observations and data were collected to understand the chemical reactions and solubility of different compounds. These observations provided insights into the reactions that occurred between the reactants and the resulting products.
First, the initial reactants were identified and their appearance was noted. This included their color, consistency, and state (solid, liquid, or gas). For example, if there were two aqueous solutions of different metal salts, the color and transparency of each solution were observed and recorded.
- Observation 1: Aqueous solution A was transparent and colorless.
- Observation 2: Aqueous solution B was yellowish and had a slight turbidity.
Next, the chemical reactions were conducted by mixing the reactants together. The changes in color, formation of precipitates, or gas evolution were closely monitored. These observations helped determine whether a reaction had occurred and what the products of the reaction were.
- Observation 3: Upon mixing solutions A and B, a pale green precipitate formed.
The final step involved determining the solubility of the products. This was done by adding various reagents to the reaction mixture and observing any changes in solubility. For example, if the precipitate formed in the reaction was insoluble in water, it could be tested for solubility in acid or base solutions.
- Observation 4: The pale green precipitate obtained from the reaction was insoluble in water but readily dissolved in a dilute acid solution.
Analysis and Interpretation of the Lab Results
The double replacement reactions and solubility lab aimed to explore the reactions that occur when two aqueous solutions are combined and to determine the solubility of various compounds. The lab focused on observing the formation of precipitates and identifying the products of the reactions.
During the lab, several pairs of solutions were mixed together, and the reaction mixtures were observed for any changes. The formation of a precipitate indicated a reaction had occurred, and the color, texture, and appearance of the precipitate helped identify the products. Additionally, the solubility of specific compounds was tested by mixing them with different solutions and observing whether they dissolved or formed a precipitate. The data collected during the lab provided valuable insights into the solubility patterns of various compounds.
The results of the lab revealed several interesting observations. For example, when silver nitrate solution was mixed with sodium chloride solution, a white precipitate of silver chloride formed. This reaction demonstrated the formation of an insoluble compound, as both silver nitrate and sodium chloride are soluble in water individually. This finding aligns with the principle that some combinations of ions form compounds that have low solubility and therefore precipitate out of solution.
Another notable result was the reaction between lead(II) nitrate solution and potassium iodide solution. A yellow precipitate of lead(II) iodide formed, indicating the formation of an insoluble compound. This reaction was particularly interesting because it demonstrated the selective precipitation of specific ions. Despite lead(II) nitrate and potassium iodide both containing ions that form insoluble compounds, only lead(II) iodide precipitated out, while potassium nitrate remained in solution.
Overall, the lab results provided valuable insights into double replacement reactions and the solubility of different compounds. The observations made during the lab helped confirm the principles and theories related to these chemical processes. The understanding gained from this lab can be applied to real-world scenarios, such as understanding how different compounds interact in environmental systems and the formation of precipitates in industrial processes.