In the study of electrochemistry, electrochemical cells play a crucial role in understanding the transfer of electrons between chemical species. These cells consist of two electrodes, namely the anode and the cathode, that are immersed in an electrolyte solution. The flow of electrons occurs as a result of redox reactions taking place at the electrodes.
Many students find worksheets on electrochemical cells challenging as they require an understanding of various concepts, including half-reactions, cell notation, and calculating cell potentials. This article aims to provide answers to common questions found in electrochemical cell worksheets, helping students grasp these complex concepts more easily.
One common question in electrochemical cell worksheets asks students to identify the half-reactions at the anode and cathode. The half-reaction at the anode is the oxidation half-reaction, where electrons are lost. Conversely, the half-reaction at the cathode is the reduction half-reaction, where electrons are gained. By identifying the species being oxidized and reduced, students can determine the half-reactions for a given electrochemical cell.
Another common question focuses on calculating the cell potential. The cell potential is a measure of the driving force behind the flow of electrons in an electrochemical cell. To calculate the cell potential, students need to determine the reduction potentials of the species involved in the half-reactions. By assigning oxidation numbers and applying the rules for balancing redox reactions, students can calculate the cell potential using the Nernst equation.
Understanding Electrochemical Cells
Electrochemical cells are devices that convert chemical energy into electrical energy through redox reactions. These cells consist of two electrodes, an anode and a cathode, which are placed in an electrolyte solution. The anode is the electrode where oxidation occurs, while the cathode is the electrode where reduction occurs.
One of the most well-known types of electrochemical cells is the battery. Batteries are portable electrochemical cells that provide energy for various devices. They are comprised of one or more cells connected in series or parallel to increase the total voltage or current output.
Types of Electrochemical Cells:
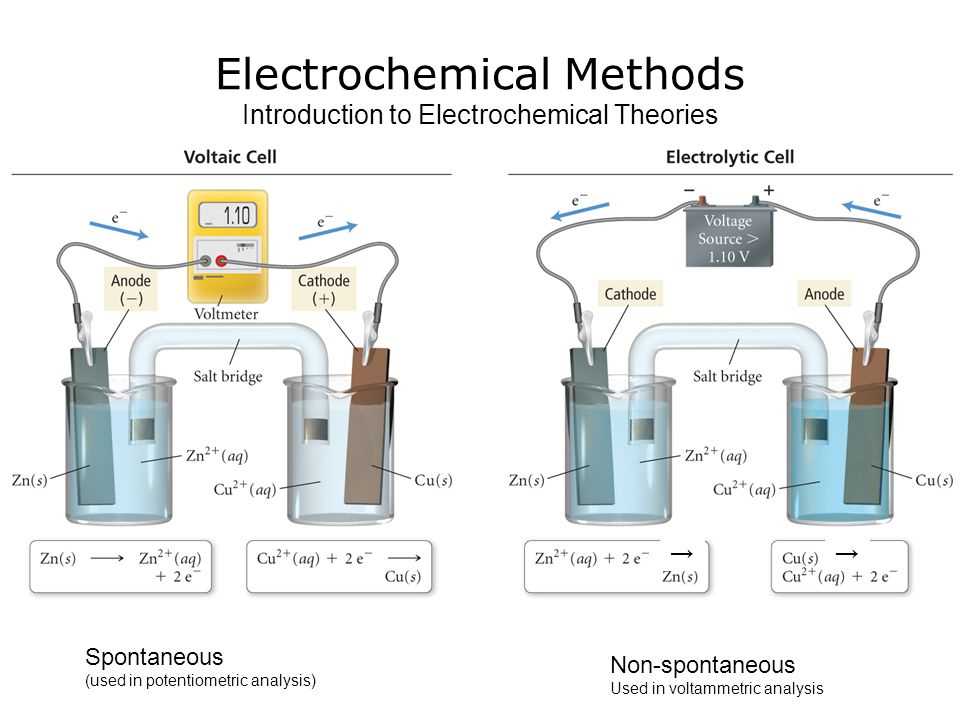
- Voltaic Cells: Also known as galvanic cells, voltaic cells use spontaneous redox reactions to produce electrical energy. The free energy change of the reaction is negative, resulting in the generation of a voltage. This allows for the continuous flow of electrons from the anode to the cathode.
- Electrolytic Cells: Electrolytic cells are used to drive non-spontaneous redox reactions by applying an external voltage. The electrolyte solution in these cells is usually an ionic compound that dissociates into ions when dissolved in water. The external voltage applied to the cell forces the movement of ions towards their respective electrodes, where oxidation and reduction occur.
Anode and Cathode Reactions:
In an electrochemical cell, oxidation occurs at the anode, where a substance loses electrons and forms ions. Reduction occurs at the cathode, where a substance gains electrons and becomes neutral or forms a compound.
| Cell Type | Anode Reaction | Cathode Reaction |
|---|---|---|
| Voltaic Cell | Oxidation | Reduction |
| Electrolytic Cell | Reduction | Oxidation |
Understanding electrochemical cells is crucial in various fields, including battery technology, corrosion prevention, and electroplating. By harnessing the potential of redox reactions, scientists and engineers can develop more efficient and sustainable energy storage systems and improve the performance of electronic devices.
What is an Electrochemical Cell?
An electrochemical cell is a device that uses chemical reactions to generate electric energy. It consists of two electrodes, an electrolyte, and a separator that prevents the electrodes from touching each other.
At one electrode, called the anode, a spontaneous oxidation reaction takes place, releasing electrons. These electrons flow through an external circuit to the other electrode, called the cathode. At the cathode, a reduction reaction occurs, consuming the electrons and completing the circuit.
The electrochemical cell is driven by the flow of ions between the electrodes through the electrolyte. The electrolyte is typically a solution or a molten salt that contains ions. These ions travel through the electrolyte to maintain charge balance and enable the flow of electrical current.
The electrochemical cell can be categorized into two types: galvanic cells and electrolytic cells.
- In a galvanic cell (also known as a voltaic cell or a battery), the chemical reaction produces electric energy, which can be used to power devices. The reaction occurs spontaneously, converting chemical energy into electrical energy.
- In an electrolytic cell, an external electric source is used to drive a non-spontaneous reaction. Electrical energy is supplied to the cell to force the reaction to occur. This allows the cell to perform processes such as electrolysis, where substances are decomposed using electrical energy.
Electrochemical cells are widely used in various applications, ranging from batteries in portable electronic devices to fuel cells for powering vehicles. They provide a convenient and efficient way to convert chemical energy into electrical energy and vice versa.
How do Electrochemical Cells Work?
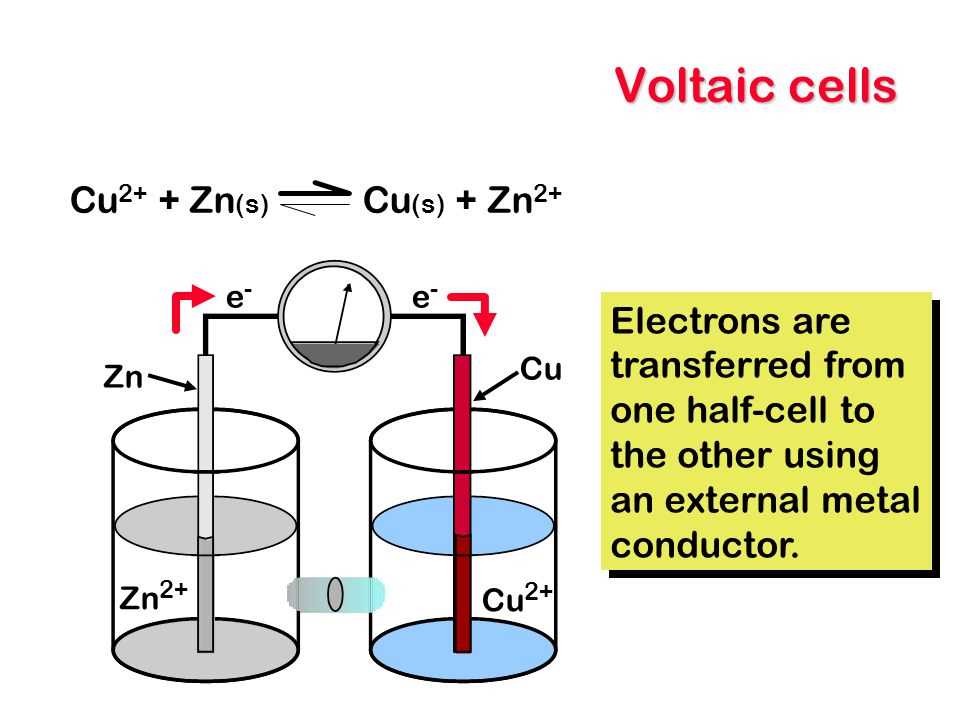
Electrochemical cells are devices that convert chemical energy into electrical energy through redox reactions. These cells consist of two half-cells, each containing an electrode immersed in an electrolyte solution. The two half-cells are connected by a pathway for the flow of ions, called the salt bridge. This allows for the transfer of electrons from one electrode to the other.
Within each half-cell, there is a redox reaction taking place. One electrode serves as the anode, where oxidation occurs, and the other electrode serves as the cathode, where reduction occurs. At the anode, oxidation leads to the generation of electrons, which flow through the external circuit to the cathode. Meanwhile, ions from the electrolyte solution move to balance the charge and maintain electrical neutrality.
The movement of electrons through the external circuit creates an electric current, which can be harnessed to power various devices. The flow of electrons from the anode to the cathode is driven by the difference in electrochemical potential between the two half-cells. This potential difference, known as cell voltage or electromotive force (EMF), is measured in volts.
Electrochemical cells can be classified into two types: galvanic cells and electrolytic cells. In galvanic cells, the redox reaction occurs spontaneously, generating electrical energy. In electrolytic cells, an external electrical source is required to drive the non-spontaneous redox reaction. These cells are important in various applications, including batteries, fuel cells, and electroplating processes.
Summary:
- Electrochemical cells convert chemical energy into electrical energy through redox reactions.
- They consist of two half-cells connected by a salt bridge.
- Oxidation occurs at the anode, generating electrons, while reduction occurs at the cathode.
- The movement of electrons through the external circuit creates an electric current.
- Cell voltage or electromotive force drives the flow of electrons.
- Galvanic cells generate electrical energy spontaneously, while electrolytic cells require an external power source.
Types of Electrochemical Cells
An electrochemical cell is a device that converts chemical energy into electrical energy by utilizing redox reactions. There are various types of electrochemical cells, each designed for specific applications and employing different configurations and electrolytes.
1. Galvanic Cell (Voltaic Cell): This type of electrochemical cell uses a spontaneous redox reaction to generate electricity. It consists of two half-cells, connected by a salt bridge or a porous barrier, and contains an electrolyte solution. The oxidation reaction occurs at the anode, releasing electrons, while the reduction reaction takes place at the cathode, accepting electrons. The flow of electrons between the anode and cathode is the source of electrical energy.
2. Electrolytic Cell: Unlike a galvanic cell, an electrolytic cell requires an external source of electrical energy to drive a non-spontaneous redox reaction. It consists of an anode and a cathode, immersed in an electrolyte solution. The anode is positively charged and acts as the site of oxidation, while the cathode is negatively charged and serves as the site of reduction. When an electric current is passed through the cell, cations migrate towards the cathode, and anions migrate towards the anode, allowing the non-spontaneous reaction to occur.
3. Fuel Cell: A fuel cell is an electrochemical device that produces electricity through the direct oxidation of a fuel. It consists of two electrodes separated by an electrolyte and requires an external source of the fuel to fuel the oxidation process. The fuel enters the anode compartment, where it is oxidized, releasing electrons. These electrons travel through an external circuit, generating electrical energy. Meanwhile, oxygen or air enters the cathode compartment and combines with the electrons and protons, forming water as a byproduct.
4. Concentration Cell: A concentration cell utilizes a gradient in the concentration of an electrolyte to generate electricity. It consists of two half-cells with the same electrode material and an electrolyte solution. The only difference between the two half-cells is the concentration of the electrolyte. One half-cell has a higher concentration, while the other has a lower concentration. The difference in concentration drives the migration of ions, creating an electrical potential difference between the two half-cells.
5. Rechargeable Cell (Secondary Cell): A rechargeable cell is an electrochemical cell that can be recharged by passing an electric current through it in the opposite direction of its discharge. This type of cell allows for the reversible conversion between chemical energy and electrical energy. Common examples include lead-acid batteries, lithium-ion batteries, and nickel-cadmium batteries. Rechargeable cells are widely used in portable electronics, electric vehicles, and renewable energy storage systems.
In conclusion, the different types of electrochemical cells offer diverse applications and functionality. They play a crucial role in various industries and technologies by providing a reliable and efficient source of electrical energy.
Voltaic Cells
Voltaic cells, also known as galvanic cells, are devices that convert chemical energy into electrical energy through a redox reaction. They consist of an anode (the electrode where oxidation occurs), a cathode (the electrode where reduction occurs), and an electrolyte solution that allows the flow of ions between the electrodes. These cells are commonly used in batteries to power various devices.
In a voltaic cell, the anode is negatively charged and the cathode is positively charged. This creates an electrical potential difference, or voltage, between the two electrodes. When a load, such as a light bulb or a motor, is connected to the cell, electrons flow from the anode to the cathode through an external circuit, generating electric current.
The redox reaction that takes place in a voltaic cell involves a transfer of electrons between the anode and the cathode. The anode undergoes oxidation, losing electrons and becoming positively charged, while the cathode undergoes reduction, gaining electrons and becoming negatively charged. This transfer of electrons occurs through the medium of the electrolyte solution, which contains ions that can move freely.
A key component of a voltaic cell is the salt bridge or the porous barrier that separates the anode and cathode compartments. This bridge allows the flow of ions between the two compartments, maintaining charge balance and preventing the build-up of excess charge. It also completes the circuit by providing a pathway for the ions to move from one electrode to the other.
- The overall reaction in a voltaic cell can be represented by a balanced chemical equation.
- The voltage of a voltaic cell can be determined using the Nernst equation.
- The efficiency of a voltaic cell is determined by factors such as the nature of the electrodes and the concentration of the electrolyte solution.
In summary, voltaic cells are devices that convert chemical energy into electrical energy through a redox reaction. They consist of an anode, a cathode, and an electrolyte solution, and are commonly used in batteries. The flow of electrons from the anode to the cathode generates electric current, which can be used to power various devices. The salt bridge or porous barrier is important for maintaining charge balance and completing the circuit. The efficiency of a voltaic cell depends on various factors and can be determined using equations such as the Nernst equation.
Electrolytic Cells
An electrolytic cell is a type of electrochemical cell that uses an external source of electrical energy to drive a non-spontaneous redox reaction. Unlike a galvanic cell, which converts chemical energy into electrical energy, an electrolytic cell requires electrical energy to initiate the reaction.
In an electrolytic cell, there are two electrodes: the anode and the cathode. The anode is connected to the positive terminal of the power source, known as the anode compartment, while the cathode is connected to the negative terminal, known as the cathode compartment. The anode is where oxidation occurs, while the cathode is where reduction takes place.
Electrolyte: The electrolyte is a solution or molten substance that contains ions. It allows the flow of electric current within the cell by facilitating the movement of ions between the anode and cathode.
Electrolytic Solution: The electrolytic solution can be a salt solution, such as sodium chloride (NaCl), or an acid solution, such as sulfuric acid (H2SO4). It provides the necessary ions for the redox reaction to occur.
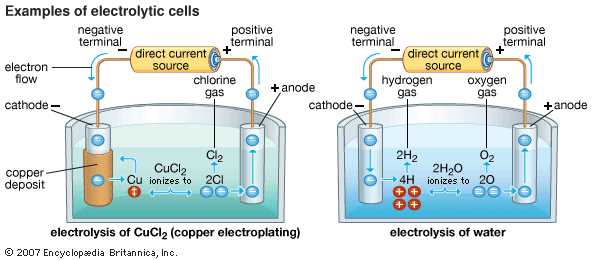
Electrolysis: The process of using electrical energy to drive a non-spontaneous redox reaction in an electrolytic cell is called electrolysis. This process is used in various applications, such as electroplating, metal extraction, and water splitting.
Electrolytic Cell Diagram: A typical diagram of an electrolytic cell includes the power source, the anode and cathode compartments, the electrolytic solution, and the external circuit connecting the electrodes to the power source.
Electrolytic Cell Examples: Examples of electrolytic cells include the electrolysis of water to produce hydrogen and oxygen gas, the electroplating of metals, and the purification of certain substances.
Overall, electrolytic cells play a crucial role in various industrial processes and scientific research, allowing for the controlled manipulation of chemical reactions using electrical energy.