
Understanding electron configuration and orbital notation is crucial in studying the behavior and properties of atoms. Worksheet 2 provides a series of exercises to help students practice and reinforce their knowledge in this area. By completing these exercises and reviewing the answers, students can further enhance their understanding of electron configurations and orbital notations.
The electron configuration of an atom refers to the distribution of electrons in its various energy levels and sublevels. Knowing the electron configuration allows us to determine the number of electrons in each shell and subshell, providing insight into the atom’s chemical behavior and its ability to form bonds. Worksheet 2 provides a range of elements and ions with their respective atomic numbers, allowing students to determine the electron configurations using the periodic table and electron configuration rules.
Orbital notation, on the other hand, represents the distribution of electrons in the different orbitals of an atom. It uses arrows to denote the electrons and their spins in each orbital. Worksheet 2 offers exercises that require students to draw orbital diagrams for various elements and ions, helping them visualize the arrangement of electrons in different energy levels and sublevels. By completing these exercises, students can further enhance their ability to interpret and analyze orbital notations.
By thoroughly studying the provided answers to Worksheet 2, students can assess their understanding of electron configurations and orbital notations. They can identify any mistakes or misconceptions they may have and seek clarification on those topics. The answers also serve as a valuable reference for students to review and reinforce their knowledge in electron configurations and orbital notations, ensuring a solid foundation in this fundamental aspect of chemistry.
Electron Configuration and Orbital Notation Worksheet 2 Answers

In Electron Configuration and Orbital Notation Worksheet 2, students were given a set of elements and asked to determine their electron configurations and orbital notations. This worksheet is designed to help students practice and reinforce their understanding of electron configurations and orbital notations, which are essential concepts in understanding the behavior and properties of elements.
To find the answers to this worksheet, students needed to apply their knowledge of the periodic table and the arrangement of electrons in energy levels and orbitals. They had to determine the number of electrons in each energy level and assign them to the appropriate orbitals based on the rules of electron configuration. By doing so, they were able to construct the electron configuration and orbital notation for each given element.
For example, for the element carbon (C), the electron configuration is 1s2 2s2 2p2. This indicates that carbon has two electrons in the 1s orbital, two electrons in the 2s orbital, and two electrons in the 2p orbital. The orbital notation for carbon would be written as ↑↑↑↑↑↑↑↓↓↓↓↓↓.
By completing this worksheet and checking their answers, students can gain a deeper understanding of electron configurations and orbital notations. They can also practice their skills in interpreting and applying the rules of electron configuration, which will be helpful in solving more complex problems in chemistry and understanding the behavior of elements in the periodic table.
Understanding Electron Configuration
Electron configuration refers to the distribution of electrons in an atom or ion. It is a descriptive model that helps us understand the arrangement of electrons in energy levels, sublevels, and orbitals. This knowledge is crucial in understanding the chemical behavior of elements and their reactions.
In electron configuration, we use a notation system that includes numbers and symbols. The numbers represent the energy levels (or shells) and the symbols represent the sublevels (s, p, d, f). The sublevels are further divided into orbitals, which can hold a maximum of two electrons with opposite spins. This notation provides a concise way to represent the electronic structure of an atom.
For example, the electron configuration of carbon (C) is 1s^2 2s^2 2p^2. This means that the carbon atom has two electrons in the 1s orbital, two electrons in the 2s orbital, and two electrons in the 2p orbital. The superscript numbers indicate the number of electrons in each orbital.
In addition to electron configuration, orbital notation is another way to represent the arrangement of electrons. In orbital notation, we use lines (or boxes) to represent orbitals and arrows to represent electrons. Each orbital can hold a maximum of two arrows, representing the two electrons with opposite spins. This notation provides a visual representation of the distribution of electrons in an atom.
Understanding electron configuration is essential in predicting the chemical properties of elements and their reactivity. It helps us determine how atoms will bond with each other to form compounds and how they will interact with other substances. By studying electron configuration, scientists can explain the periodic trends in the periodic table and make predictions about the behavior of elements in chemical reactions.
Exploring Orbital Notation
Orbital notation is a way of representing the arrangement of electrons in an atom’s orbitals. It is a visual representation that uses arrows to represent electrons and boxes or lines to represent orbitals. By understanding orbital notation, we can gain insights into the electron configuration and the properties of an atom.
Key Features of Orbital Notation:
- Orbitals: Orbitals are regions in space where electrons are likely to be found. They are represented by boxes or lines in orbital notation.
- Arrows: Arrows are used to represent electrons. Upward arrows (↑) indicate electrons with positive spin, while downward arrows (↓) represent electrons with negative spin.
- Pauli Exclusion Principle: The Pauli Exclusion Principle states that no two electrons in an atom can have the same set of four quantum numbers. This principle is reflected in orbital notation by placing the arrows in separate boxes or lines within the same orbital.
Orbital notation allows us to visualize the electron configuration of atoms, which in turn determines their chemical behavior and properties. By understanding the arrangement of electrons in different orbitals, we can predict an atom’s reactivity, bonding behavior, and even its color and magnetism.
Examples of Orbital Notation:
| Element | Electron Configuration | Orbital Notation |
|---|---|---|
| Lithium (Li) | 1s2 2s1 | ↑↓ | ↑ |
| Oxygen (O) | 1s2 2s2 2p4 | ↑↓ | ↑↓ | ↑↓↑↓ | ↑ |
| Fluorine (F) | 1s2 2s2 2p5 | ↑↓ | ↑↓ | ↑↓↑↓ | ↑↓ |
Overall, orbital notation is a powerful tool for understanding the electron configuration and properties of atoms. It allows us to predict an atom’s behavior and provides a visual representation of the arrangement of electrons in an atom’s orbitals.
Reviewing the Basics

Understanding electron configuration and orbital notation is crucial when studying the properties and behavior of atoms and molecules. This review will cover some basic concepts and help solidify your understanding of these topics.
Electron configuration is a way of representing how electrons are distributed in a atom’s energy levels or shells. The electron configurations are written using the periodic table as a guide, with the number of electrons in each shell indicated by superscript numbers. For example, the electron configuration of carbon is 1s^2 2s^2 2p^2, which shows that carbon has 2 electrons in the first energy level, 2 electrons in the second energy level, and 2 electrons in the third energy level.
Orbital notation is another way of representing the distribution of electrons in an atom. In orbital notation, each electron is represented by an arrow. The direction of the arrow indicates the spin of the electron (up or down), and the level and type of the orbital are indicated by the box or line where the arrow is placed. For example, the orbital notation for carbon would be represented by two arrows pointing up in the 1s orbital, two arrows pointing up in the 2s orbital, and two arrows pointing up in the 2p orbital.
To determine the electron configuration of an atom, you can use the Aufbau principle, which states that electrons fill the lowest energy levels first before moving to higher energy levels. The Pauli exclusion principle states that each orbital can hold a maximum of two electrons with opposite spins, while the Hund’s rule states that electrons fill each orbital singly before pairing up with opposite spins.
Understanding and applying these principles will allow you to accurately represent an atom’s electron configuration and orbital notation, which in turn will help you predict its chemical behavior and understand its properties.
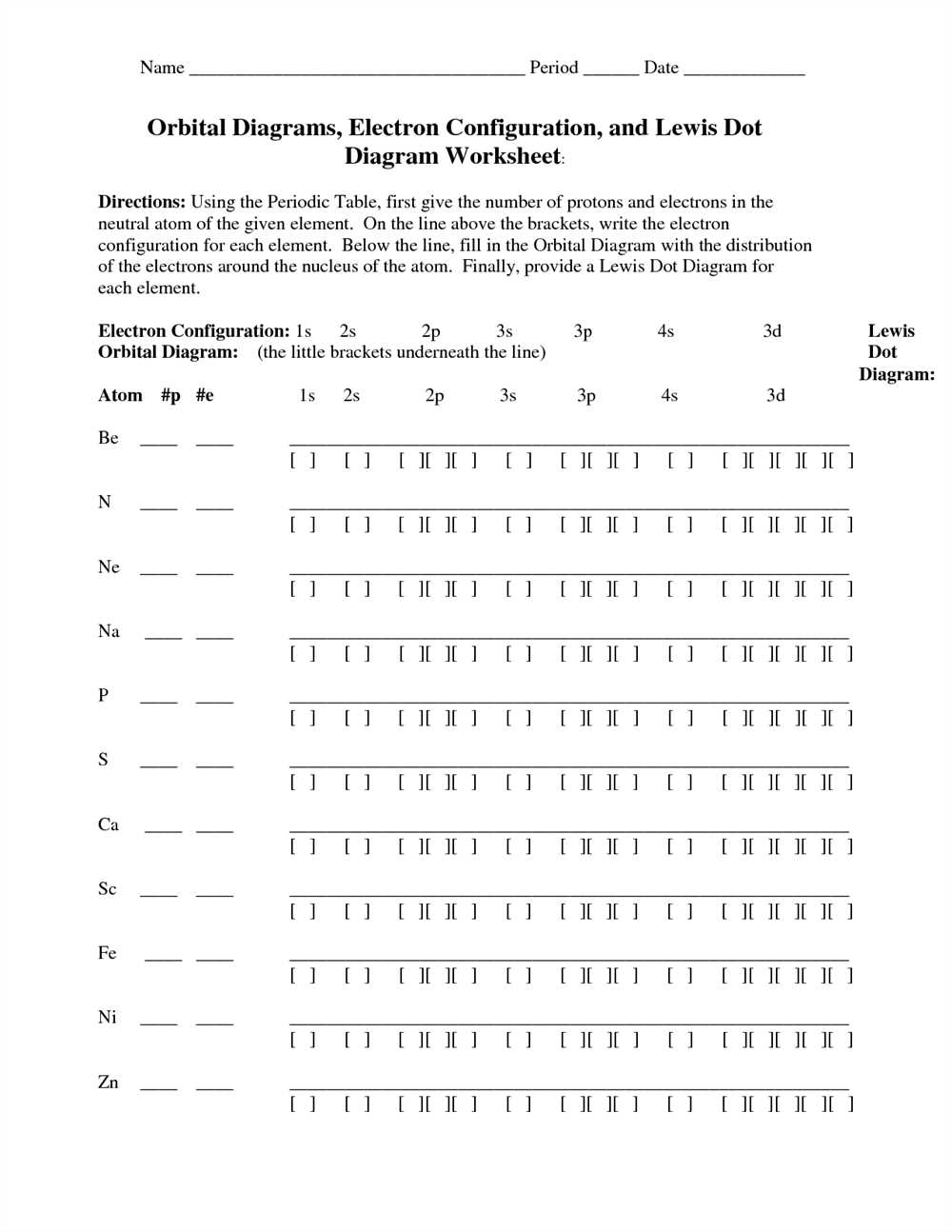
Electron Configuration Worksheet 2: Problem Set
In the study of chemistry, understanding the electron configuration of an atom is crucial to predicting its chemical behavior and properties. The electron configuration describes the arrangement of electrons in the energy levels and sublevels of an atom. It provides a roadmap for understanding the distribution of electrons and their relationship to the periodic table.
The Electron Configuration Worksheet 2: Problem Set serves as a valuable tool for students to practice and reinforce their understanding of electron configuration. This worksheet presents a series of problems that challenge students to determine the electron configuration and orbital notation of various elements. By solving these problems, students can enhance their skills in interpreting electron configurations and further solidify their knowledge of atomic structure.
The problem set includes a variety of elements, representing different periods and groups in the periodic table. Each element is accompanied by a set of questions that guide students through the process of determining its electron configuration. Through this exercise, students become familiar with the concepts of energy levels, sublevels, orbitals, and electron spin.
The Electron Configuration Worksheet 2: Problem Set encourages students to apply their knowledge of electron configuration in a practical setting. By actively engaging in problem-solving, students develop critical thinking skills and gain confidence in their ability to analyze and interpret electron configurations. This worksheet is an invaluable resource for chemistry students seeking to master the complexities of electron configuration.
Key Takeaways
- The electron configuration of an atom describes the arrangement of electrons in its energy levels and sublevels.
- The Electron Configuration Worksheet 2: Problem Set helps students practice determining electron configurations and orbital notation.
- By solving the problems in this worksheet, students enhance their understanding of atomic structure and the periodic table.
- The problem set includes a variety of elements, allowing students to explore different periods and groups in the periodic table.
- This worksheet encourages active problem-solving and critical thinking skills.
How to Determine Electron Configuration
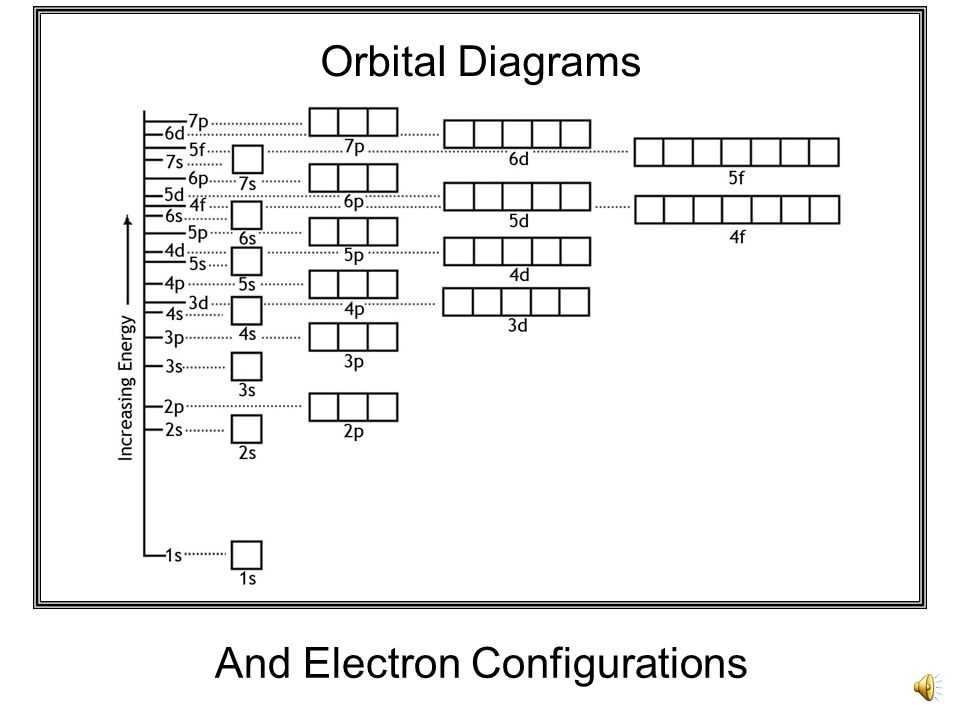
In chemistry, electron configuration refers to the arrangement of electrons in an atom or molecule. It provides valuable information about an element’s physical and chemical properties. To determine the electron configuration for an atom, you need to follow a specific set of rules and principles.
1. Aufbau Principle: This principle states that electrons fill the lowest energy levels first before moving on to higher energy levels. The energy levels are represented by the numbers 1, 2, 3, and so on, known as the principal quantum numbers.
2. Pauli Exclusion Principle: According to this principle, no two electrons in an atom can have the same set of quantum numbers. This means that each electron must have a unique combination of the principal quantum number, azimuthal quantum number, magnetic quantum number, and spin quantum number.
3. Hund’s Rule: Hund’s rule states that when multiple orbitals of the same energy level are available, electrons will occupy separate orbitals with parallel spins before filling the orbitals with opposite spins. This principle helps to maximize the stability of the atom.
To write the electron configuration of an atom, you start by listing the electron shells and subshells in order of increasing energy. For example, the electron configuration of carbon is 1s^2 2s^2 2p^2, indicating that carbon has two electrons in the 1s subshell, two electrons in the 2s subshell, and two electrons in the 2p subshell.
You can also use the periodic table to determine the electron configuration. The periodic table is organized in a way that shows the pattern of filling electron shells and subshells. By following this pattern, you can easily write the electron configuration of any element.
Overall, determining electron configuration is essential for understanding an element’s behavior and reactivity. It allows chemists to predict chemical reactions, bonding patterns, and physical properties, making it a crucial concept in chemistry.
Applying Hund’s Rule and the Aufbau Principle
When determining the electron configuration of an atom, it is important to apply Hund’s Rule and the Aufbau Principle. These rules help us understand how electrons fill up the different energy levels and orbitals in an atom.
The Aufbau Principle states that electrons occupy the lowest energy levels and orbitals first before filling up the higher energy levels. Essentially, electrons fill up the orbitals in a specific order, starting from the lowest energy level and moving up sequentially.
To apply Hund’s Rule, we must understand that each orbital can accommodate a maximum of two electrons. When filling up orbitals within the same energy level, electrons will first occupy separate orbitals before pairing up. This means that if there are two or more empty orbitals within the same energy level, electrons will fill up those orbitals with parallel spins before pairing up.
For example, let’s consider the electron configuration of nitrogen. Nitrogen has an atomic number of 7, which means it has 7 electrons. Following the Aufbau Principle, the first two electrons will fill the 1s orbital, the next two will fill the 2s orbital, and the remaining three will fill the 2p orbitals. When applying Hund’s Rule, we can see that the three electrons in the 2p orbitals will each occupy a separate orbital with parallel spins before pairing up.
In conclusion, by applying Hund’s Rule and the Aufbau Principle, we can determine the order in which electrons occupy different energy levels and orbitals in an atom’s electron configuration. This helps us understand the distribution of electrons and their interactions within an atom.