
Understanding electron configuration is crucial in comprehending chemical reactions and properties of elements. By learning the arrangement of electrons in an atom, scientists can unlock the secrets of an element’s behavior. To aid in this learning process, electron configuration orbital diagrams worksheets are often used in chemistry education. These worksheets provide practice in drawing orbital diagrams and help students identify the distribution of electrons in different energy levels.
The answer key for electron configuration orbital diagrams worksheets serves as a valuable resource for students and teachers alike. It offers a step-by-step guide to determining the correct electron arrangement for each element. By understanding the principles behind electron configuration, students can gain a deeper understanding of the periodic table and the organization of elements.
The worksheet answer key not only provides the correct answers but also offers explanations and insights into why certain electron configurations are preferred. This allows students to develop a conceptual understanding of orbital diagrams and electron distribution patterns. Additionally, the answer key helps students identify common pitfalls and misconceptions, enabling them to correct any mistakes and solidify their understanding of electron arrangement.
Overall, the electron configuration orbital diagrams worksheet answer key is an essential tool for students studying chemistry. It provides a comprehensive guide to electron arrangement, allowing students to develop a deep understanding of the periodic table and the behavior of different elements. With this knowledge, students can tackle more complex concepts in chemistry and gain a solid foundation for future scientific endeavors.
Overview of Electron Configuration Orbital Diagrams Worksheet

The Electron Configuration Orbital Diagrams Worksheet is a valuable tool for understanding the arrangement of electrons in an atom or ion. It provides a visual representation of the distribution of electrons in different energy levels and orbitals. This worksheet is often used in chemistry and physics education to help students grasp key concepts related to electron configurations.
The worksheet consists of several sections, each focusing on a specific aspect of electron configuration. These sections typically include instructions, examples, and practice problems. Students are tasked with filling in the blanks and completing orbital diagrams based on the given information. The worksheet may also include questions that require students to identify the element or ion based on its electron configuration.
The electron configuration orbital diagrams worksheet is designed to help students understand the relationship between orbitals, energy levels, and the filling order of electrons. It encourages critical thinking and problem-solving skills as students analyze and interpret the information provided. By using the worksheet, students gain a deeper understanding of electron configurations and how they contribute to the chemical properties of an element or ion.
Overall, the Electron Configuration Orbital Diagrams Worksheet serves as a valuable educational resource for students studying chemistry and physics. It provides a hands-on approach to learning and reinforces key concepts related to electron configurations. By completing this worksheet, students develop a solid foundation in understanding the arrangement of electrons in atoms and ions, which is essential for further studies in chemistry and other related fields.
What is an Electron Configuration Orbital Diagram?
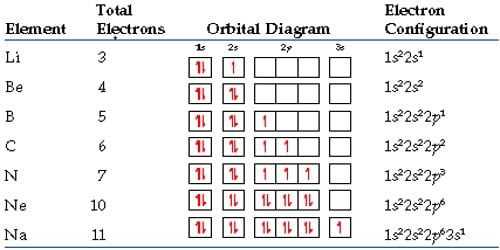
An electron configuration orbital diagram is a visual representation of the arrangement of electrons in an atom’s orbitals. It helps in determining the distribution of electrons in different energy levels and subshells of an atom. The diagram displays the orbitals as boxes, with arrows representing the electrons. This diagram provides a concise way to represent the electron configuration of an element.
The electron configuration of an atom describes the arrangement of electrons in its energy levels and subshells. It follows a specific pattern based on the Aufbau principle, Pauli exclusion principle, and Hund’s rule. The Aufbau principle states that lower-energy orbitals are filled before higher-energy ones, and the Pauli exclusion principle states that each orbital can accommodate a maximum of two electrons with opposite spins. Hund’s rule states that when orbitals of equal energy are available, electrons fill them singly before pairing up.
The electron configuration orbital diagram shows the different energy levels and subshells of an atom. The energy levels are represented by the rows in the diagram, labeled as 1, 2, 3, etc. The subshells, such as s, p, d, and f, are represented by the columns. Each box in the diagram represents an orbital, and the arrows within the boxes represent the electrons. The direction of the arrow represents the electron spin, with one arrow pointing up and the other down to indicate opposite spins.
An electron configuration orbital diagram provides a visual representation of the electron arrangement, making it easier to understand and analyze the properties and behavior of an atom. It is a useful tool in chemistry and physics to predict the chemical reactivity and bonding of elements, as well as understand their electronic structure and energy levels. By studying the electron configuration orbital diagrams, scientists can gain insights into the behavior of elements and their interactions with other substances.
Importance of Electron Configuration in Chemistry
Electron configuration is a fundamental concept in chemistry that describes the arrangement of electrons within an atom. It is significant because it determines the chemical behavior and properties of elements. By understanding the electron configuration, scientists can predict and explain various chemical reactions and the formation of chemical bonds.
Electron configuration is based on the principle that each electron within an atom occupies a specific energy level and sublevel. The arrangement of electrons in these energy levels and sublevels is dictated by the Pauli exclusion principle, which states that no two electrons can have the same set of quantum numbers. The Aufbau principle, on the other hand, states that electrons occupy the lowest available energy levels before filling higher energy levels.
Knowing the electron configuration of an element allows scientists to determine its reactivity and chemical properties. Elements with similar electron configurations tend to exhibit similar chemical behavior. For example, the noble gases have full valence electron shells, making them stable and unreactive. Transition metals, on the other hand, have incompletely filled d orbitals, which allow them to form a variety of different oxidation states and exhibit catalytic properties.
Electron configuration also plays a crucial role in understanding and predicting the formation of chemical bonds. The concept of valence electrons, which are the outermost electrons in an atom, is directly related to electron configuration. Elements with similar electron configurations tend to have similar numbers of valence electrons and therefore exhibit similar bonding patterns. The octet rule, for instance, predicts that atoms tend to gain, lose, or share electrons in order to achieve a stable configuration with eight valence electrons.
In summary, electron configuration is a fundamental concept in chemistry that determines the chemical behavior and properties of elements. By understanding and analyzing electron configurations, scientists can predict and explain chemical reactions, the formation of chemical bonds, and the behavior of different elements in various chemical environments.
Understanding Electron Configuration Orbital Diagrams
Electron configuration is an important concept in the field of chemistry, as it describes how electrons are distributed among the energy levels and orbitals in an atom or ion. The arrangement of electrons in an atom determines its chemical properties and behavior. To represent electron configuration, orbital diagrams are commonly used.
Orbital diagrams provide a visual representation of the distribution of electrons in the different energy levels and orbitals of an atom. Each energy level is represented by a numbered row, and each orbital within a given energy level is represented by a box. The arrows within the boxes indicate the direction of electron spin. The number of boxes in each energy level corresponds to the maximum number of electrons that can occupy that level according to the Aufbau principle.
The Aufbau principle states that electrons fill the lowest energy levels and orbitals first before moving to higher energy levels. This means that each energy level is filled in a specific order, with the s sublevel filling before the p sublevel, and so on. The orbitals within a sublevel are filled one at a time, with electrons pairing up in opposite spins until each orbital within the sublevel is occupied.
Understanding electron configuration and orbital diagrams is crucial for predicting and explaining the chemical behavior of elements and compounds. It allows scientists to determine the valence electrons, which are the electrons involved in chemical bonding, as well as the stability and reactivity of different atoms and ions. It also provides a basis for understanding periodic trends and the formation of chemical bonds.
In conclusion, electron configuration and orbital diagrams play a fundamental role in the study of chemistry. By representing the distribution of electrons in atoms, they provide a visual tool for understanding the arrangement of electrons and their influence on the chemical properties of elements and compounds.
The Aufbau Principle
The Aufbau Principle is a fundamental concept in chemistry that describes how electrons are added to atomic orbitals in a specific order to build the electron configuration of an atom. This principle is based on the idea that electrons occupy the lowest-energy orbitals available before filling higher-energy orbitals.
The Aufbau Principle can be understood by considering the arrangement of electrons in the periodic table. Each row in the periodic table represents a new energy level, or shell, and each column represents a new type of orbital. According to the Aufbau Principle, electrons fill the orbitals in a specific order: starting with the lowest-energy orbital and moving up in energy as more electrons are added.
The order in which electrons occupy the orbitals is determined by the energy levels and the shapes of the orbitals. The lowest-energy orbital, called the 1s orbital, is filled first, followed by the 2s and 2p orbitals. The 2s orbital is lower in energy than the 2p orbitals, so it is filled before the 2p orbitals.
The Aufbau Principle helps to explain why the electron configuration of an atom can be determined based on its position in the periodic table. By following the order of filling orbitals dictated by the Aufbau Principle, one can easily determine the electron configuration of any atom and understand its chemical behavior.
Hund’s Rule
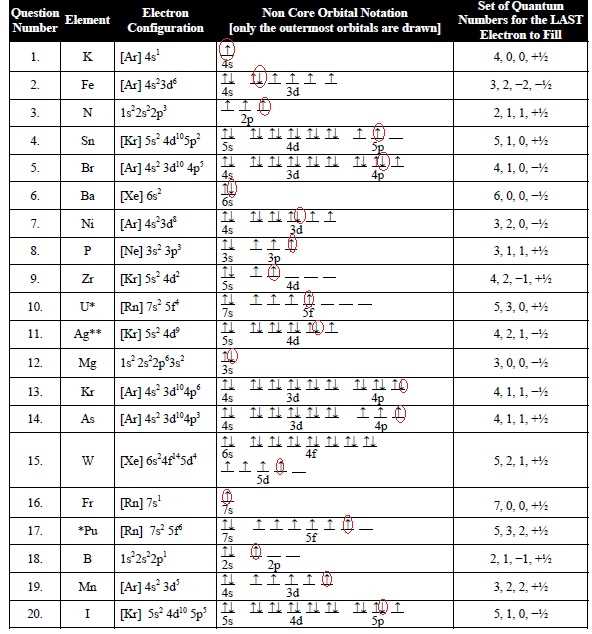
Hund’s Rule is a fundamental principle in quantum mechanics that describes how electrons fill up atomic orbitals. According to Hund’s Rule, electrons will occupy empty orbitals of the same energy level before pairing up with electrons in the same orbital. This rule is based on the concept of electron spin, where each electron can have either an “up” or “down” spin.
In other words, Hund’s Rule states that electrons will spread out across the orbitals in a sublevel, rather than pairing up, in order to maximize their overall spin alignment. This means that when filling up orbitals in the same energy level, each orbital will be occupied by a single electron before any pairing occurs.
For example, let’s consider the electron configuration of nitrogen, which has atomic number 7. Nitrogen’s electron configuration can be represented as 1s^2 2s^2 2p^3. According to Hund’s Rule, the three electrons in the 2p sublevel will fill up the three available orbitals (2p_x, 2p_y, and 2p_z) with a single electron in each orbital, before pairing up.
Hund’s Rule is crucial in understanding the behavior and properties of atoms. It helps explain phenomena such as the magnetic properties of materials and the stability of certain electron configurations. By following Hund’s Rule, scientists can accurately predict the electron arrangements and energy levels of different elements and molecules, providing valuable insights into their chemical and physical properties.
Pauli Exclusion Principle
The Pauli Exclusion Principle is a fundamental principle in quantum mechanics that governs the behavior of electrons in an atom. It states that no two electrons in an atom can have the same set of quantum numbers. This means that each electron must have a unique combination of values for its principal quantum number (n), azimuthal quantum number (l), magnetic quantum number (m), and spin quantum number (s).
This principle plays a crucial role in determining the electron configuration and orbital diagrams of atoms. The electron configuration describes how the electrons are distributed among the energy levels and orbitals of an atom. The orbital diagrams, on the other hand, visually represent the arrangement of electrons in different orbitals using arrows.
The Pauli Exclusion Principle ensures that each orbital can hold a maximum of two electrons, with opposite spins (up and down). This means that the first electron in an orbital must have a spin of +1/2, while the second electron must have a spin of -1/2. This results in the filling order of energy levels and orbitals proposed by the Aufbau principle.
- Principal quantum number (n): Determines the energy level of the electron.
- Azimuthal quantum number (l): Describes the shape of the orbital.
- Magnetic quantum number (m): Specifies the orientation of the orbital within a subshell.
- Spin quantum number (s): Represents the direction of electron spin.
By following the Pauli Exclusion Principle, we can accurately determine the electron configurations and orbital diagrams of atoms, allowing us to understand their chemical properties and behavior.