
If you love chemistry and are interested in learning more about electron configuration, then you have probably come across word find puzzles as a fun way to test your knowledge. These puzzles challenge you to find different electron configuration terms hidden within a grid of letters. However, sometimes these puzzles can be challenging, leaving you searching for the answers. That’s where this electron configuration word find answer key comes in handy.
In this article, we will provide you with the answers to common electron configuration word find puzzles. Instead of spending hours trying to figure out where those elusive terms are hidden, you can refer to our answer key to quickly find the solutions. Whether you are a student studying electron configuration or a chemistry enthusiast looking for a fun challenge, this answer key will help you complete your word find puzzles with ease.
The electron configuration word find answer key includes terms such as s sublevel, p sublevel, d sublevel, f sublevel, orbital, electron, nucleus, valence, energy level, and many more. With this comprehensive answer key, you can quickly locate these terms within the word find puzzles and solve them in no time.
So, whether you are a teacher looking for a resource to help your students or an individual wanting to sharpen their knowledge of electron configuration, this answer key is the perfect tool. It provides a convenient and time-saving solution to finding the answers to electron configuration word find puzzles, allowing you to focus on learning and enjoying the world of chemistry.
Understanding Electron Configuration
Electron configuration is a fundamental concept in chemistry that describes the distribution of electrons in an atom. It is crucial for understanding the properties and behavior of atoms, as it determines how these atoms interact with other atoms and molecules. The electron configuration of an atom is represented by a series of numbers and letters that indicate the energy levels, sublevels, and number of electrons in each sublevel.
The electron configuration follows a specific pattern based on the order of filling the energy levels and sublevels. The order of filling is determined by the Aufbau principle, which states that electrons occupy the lowest energy levels and sublevels first before filling the higher ones. This principle helps explain why certain elements have similar properties and trends in the periodic table, as their electron configurations follow similar patterns.
The electron configuration is typically written using the notation: 1s2 2s2 2p6 3s2 3p6, and so on. The numbers represent the energy levels (n), while the letters and superscripts represent the sublevels (s, p, d, f) and the number of electrons in each sublevel, respectively.
The electron configuration provides information about the stability and reactivity of atoms. For example, atoms with full sublevels are more stable and less likely to form chemical bonds, while atoms with partially filled sublevels are more reactive and tend to form bonds with other atoms to achieve a stable electron configuration.
In conclusion, understanding electron configuration is essential for understanding the behavior and properties of atoms. It helps explain trends in the periodic table, provides insights into chemical bonding, and gives valuable information about the stability and reactivity of atoms. By studying electron configuration, scientists can better comprehend the intricacies of chemical reactions and the nature of matter.
What is Electron Configuration?
Electron configuration refers to the arrangement of electrons within an atom or ion. It describes how the electrons are distributed among the available energy levels and atomic orbitals. This arrangement determines the chemical properties and behavior of an element.
Electrons occupy different energy levels and sublevels around the nucleus of an atom. The energy levels, often represented by the principal quantum number (n), are organized into several sublevels, each characterized by a specific shape and orientation. These sublevels are identified by letters – s, p, d, and f.
In electron configuration, the principle of Aufbau’s principle is followed. It states that electrons fill the available energy levels and sublevels in a specific order, starting from the lowest energy level and sublevel. The order of filling is typically s → p → d → f. Each sublevel can hold a certain maximum number of electrons – s sublevel can hold a maximum of 2 electrons, p sublevel can hold a maximum of 6 electrons, d sublevel can hold a maximum of 10 electrons, and f sublevel can hold a maximum of 14 electrons.
Electron configuration is often represented in a shortened format using the noble gas notation. The noble gas notation involves using the symbol for the nearest noble gas element with a lower atomic number in square brackets, followed by the configuration of the remaining electrons. This notation helps simplify the representation of electron configurations for elements with larger atomic numbers.
In summary, electron configuration is a way to describe how electrons are arranged within an atom or ion. It follows specific rules and principles and helps determine the chemical properties and behavior of elements. The noble gas notation is often used to simplify the electron configuration representation.
Importance of Electron Configuration
Electron configuration refers to the arrangement of electrons in an atom, specifically in the shells and subshells of an atom’s energy levels. It plays a crucial role in determining the chemical properties and behavior of elements.
The electron configuration of an atom directly affects its reactivity and ability to form chemical compounds. Understanding the electron configuration allows scientists to predict the element’s behavior in various chemical reactions. For example, the number of valence electrons, which are found in the outermost energy level, determines an element’s ability to bond with other atoms. Elements with a full outer energy level, such as noble gases, tend to be stable and unreactive, while elements with few valence electrons are highly reactive.
The electron configuration also provides valuable information about an element’s size and shape. The arrangement of electrons in different energy levels creates distinct patterns, which can be represented using electron configuration diagrams. These diagrams help visualize the spatial arrangement of electrons and their probability densities, giving insight into an atom’s size and shape.
In addition, electron configuration is essential for understanding the periodic table and its organization. The periodic table is arranged based on the electron configurations of elements, which allows for the classification of elements into groups and periods. Elements within the same group often have similar electron configurations and share similar chemical properties. The periodic table’s structure helps scientists make predictions about the properties of newly discovered elements and their placement in the table.
In summary, electron configuration is an important concept in chemistry that provides crucial information about an element’s reactivity, size, shape, and placement in the periodic table. It enables scientists to understand and predict the behavior of elements in various chemical reactions and contributes to our overall knowledge of the building blocks of matter.
Electron Configuration Notation
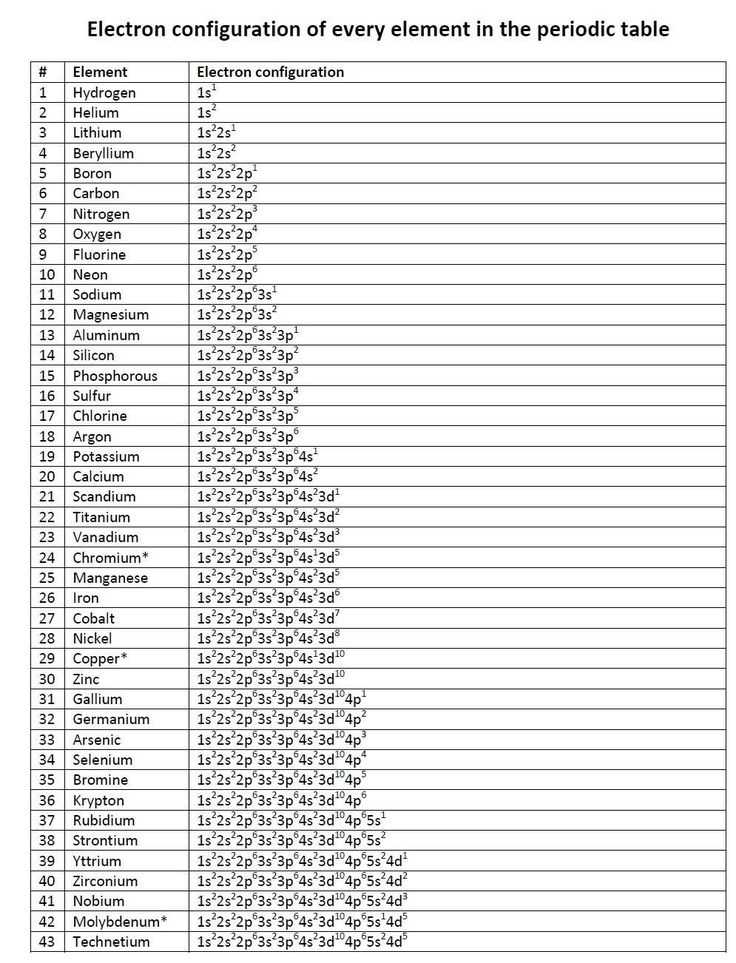
The electron configuration notation is a way to represent the distribution of electrons in an atom. It provides a concise and systematic way to describe the arrangement of electrons in the atomic orbitals of an atom.
In electron configuration notation, the shells and subshells are represented by numbers and letters. The number represents the principal quantum number, which indicates the energy level of the electron, and the letter represents the subshell, which indicates the type of orbital the electron occupies.
The electron configuration notation follows a specific order of filling orbitals, known as the Aufbau principle. According to this principle, electrons fill the orbitals of an atom in a way that minimizes their energy. The order of filling is as follows: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, and 7p.
The electron configuration notation uses superscripts to represent the number of electrons in each subshell. For example, the electron configuration of carbon (6 electrons) is 1s^2 2s^2 2p^2. The superscripts indicate that there are 2 electrons in the 1s subshell, 2 electrons in the 2s subshell, and 2 electrons in the 2p subshell.
The electron configuration notation is essential in understanding the properties and behavior of atoms. It helps to determine the stability and reactivity of elements and provides insight into their chemical bonding and structure. By using electron configuration notation, scientists can make predictions about the behavior of atoms and how they interact with other atoms.
Ground State vs. Excited State Electron Configurations
In an atom, electrons occupy specific energy levels, or orbitals, around the nucleus. The distribution of electrons within these orbitals determines the electron configuration of the atom. The electron configuration describes the arrangement of electrons in the various energy levels and sublevels.
The ground state electron configuration refers to the lowest energy arrangement of electrons in an atom. In this state, the electrons fill the orbitals starting from the lowest energy level and moving upwards. The electrons follow the Aufbau principle, which states that the orbitals are filled in order of increasing energy. For example, the electron configuration of carbon in the ground state is 1s^2 2s^2 2p^2, where electrons fill the 1s orbital first, then the 2s orbital, and finally the 2p orbital.
When an atom absorbs energy, such as through the absorption of light, its electrons can move to higher energy levels. This results in an excited state electron configuration. In the excited state, one or more electrons occupy higher energy orbitals than the ground state configuration. For example, the excited state electron configuration of carbon can be represented as 1s^2 2s^1 2p^3, where one electron from the 2s orbital has moved to the 2p orbital.
Excited state electron configurations are temporary and unstable, as the electrons in higher energy levels are prone to releasing energy and returning to their ground state configuration. When electrons return to lower energy levels, they emit photons with specific wavelengths, resulting in the emission of light. This phenomenon is observed in fireworks displays or neon lights, where excited state electrons within atoms emit light of different colors as they return to their ground state configurations.
In summary, the ground state electron configuration represents the lowest energy arrangement of electrons within an atom, while excited state electron configurations occur when electrons absorb energy and occupy higher energy orbitals. The transition from excited state to ground state results in the emission of light with specific wavelengths.
Orbital Filling Diagram Method
The orbital filling diagram method is a visual representation of the electrons in an atom’s orbitals. It is a way to show how the electrons fill up the orbitals in a specific order, following the rules of the Pauli exclusion principle and Hund’s rule.
In the orbital filling diagram, each orbital is represented by a box, and the arrows inside the boxes represent the electrons. The boxes are arranged in order of increasing energy levels, with the lower energy orbitals filled first. The arrow pointing up represents an electron with a positive spin, while the arrow pointing down represents an electron with a negative spin.
The orbital filling diagram method can be used to determine the electron configuration of an atom. The electron configuration describes the distribution of the electrons in the atomic orbitals of an atom. By following the rules of filling up the orbitals, one can determine the electron configuration for any element of the periodic table.
For example, let’s take the element carbon (C) with an atomic number of 6. The orbital filling diagram for carbon would start with the 1s orbital, which can hold up to 2 electrons. The first arrow would point up in the 1s orbital, representing the first electron. The second electron would also go in the 1s orbital, represented by a second arrow pointing down. Next, the 2s orbital would be filled with two electrons, one pointing up and one pointing down. Finally, the three remaining electrons would go in the 2p orbitals, with one electron in each orbital, all pointing up.
The orbital filling diagram method provides a clear and organized way to represent the electron configuration of atoms. It helps in understanding the arrangement of electrons and the properties of elements based on their electron configurations.
Noble Gas Notation Method
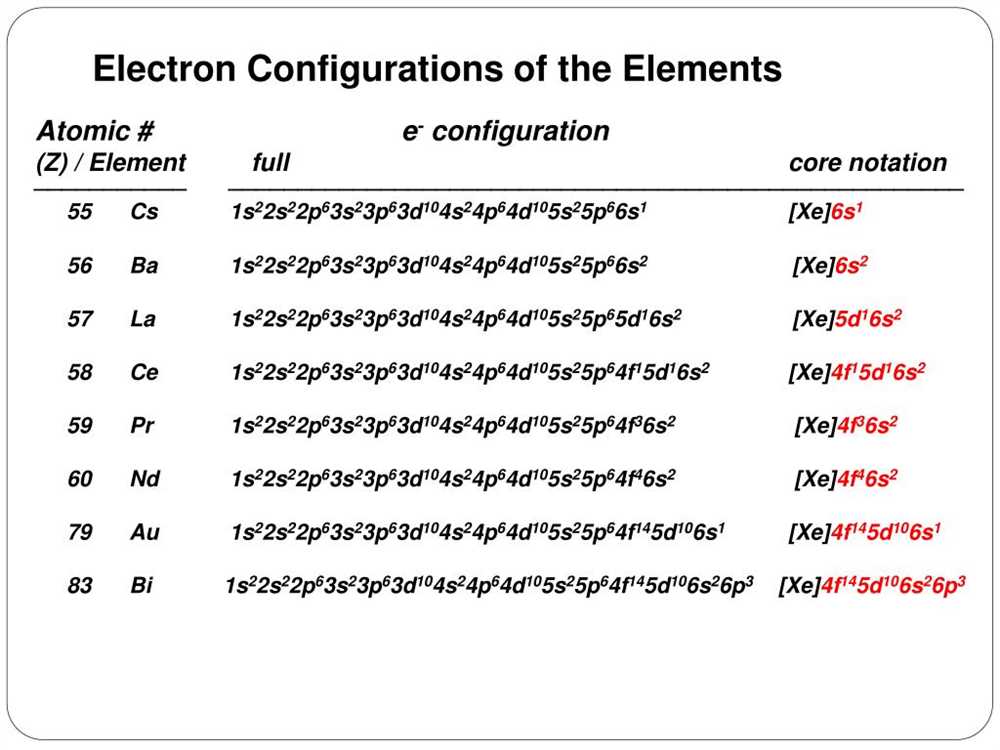
The Noble Gas Notation method is a shorthand method used to represent the electron configuration of an atom. It is named after the noble gases, which have full valence electron shells and stable electronic configurations. The Noble Gas Notation method allows us to indicate the electron configuration of an element by using the noble gas closest in atomic number below it.
The electron configuration of an element is the arrangement of its electrons in the various atomic orbitals of its atoms. It consists of a series of numbers and letters that represent the specific energy levels, sublevels, and the number of electrons in each sublevel. The electron configuration can be written in long form or in shorthand using the Noble Gas Notation method.
In the Noble Gas Notation method, the electron configuration of the noble gas is written first, enclosed in brackets. This represents the full valence electron shell of the noble gas. Following the noble gas notation, the remaining electron configuration of the element is written. For example, the electron configuration of sulfur (S) can be written as [Ne] 3s2 3p4, where [Ne] represents the electron configuration of neon.
The Noble Gas Notation method is a convenient way to represent the electron configuration of an element, especially for elements with larger atomic numbers. It helps to simplify the notation and make it easier to understand and compare the electron configurations of different elements. This method is widely used in chemistry and is an important tool for understanding the behavior and properties of elements.