
An endothermic reaction is a chemical reaction that absorbs heat energy from its surroundings. In other words, it “takes in” heat from the environment. The reactants of the endothermic reaction have a lower energy level than the products, meaning that energy needs to be added for the reaction to occur. This energy is usually in the form of heat, but it can also come from other sources such as light or electricity.
On the other hand, an exothermic reaction is a chemical reaction that releases heat energy into its surroundings. This means that it “gives out” heat to the environment. The reactants of the exothermic reaction have a higher energy level than the products, so energy is released as the reaction takes place. This energy is usually in the form of heat, but it can also be released in the form of light, sound, or electricity.
When comparing endothermic and exothermic reactions, it is important to understand the key differences between them. In an endothermic reaction, the temperature of the surroundings decreases because heat is being absorbed. This means that the surroundings feel colder. On the other hand, in an exothermic reaction, the temperature of the surroundings increases because heat is being released. This means that the surroundings feel warmer.
Understanding endothermic and exothermic reactions is essential in many areas of science, including chemistry and thermodynamics. By studying these reactions, scientists can better understand how energy is transferred and transformed in chemical systems. It also has practical applications, such as the design of heat exchangers or the development of energy-efficient processes. Answering worksheets on this topic helps students reinforce their understanding of these concepts and apply them to real-life situations.
What are endothermic and exothermic reactions?
An endothermic reaction is a type of chemical reaction that absorbs heat from its surroundings. This means that the reaction requires an input of energy in order to proceed. The energy is usually absorbed in the form of heat, but it can also be in the form of light or electricity. Endothermic reactions are characterized by a decrease in temperature and an increase in the entropy of the system. Some examples of endothermic reactions include the reaction between baking soda and vinegar, the dissolution of ammonium nitrate in water, and the evaporation of water.
In contrast, an exothermic reaction is a type of chemical reaction that releases heat to its surroundings. This means that the reaction gives off energy as it proceeds. The energy is usually released in the form of heat, but it can also be in the form of light or sound. Exothermic reactions are characterized by an increase in temperature and a decrease in the entropy of the system. Some examples of exothermic reactions include the combustion of gasoline, the reaction between sodium and water, and the formation of rust.
It is important to note that the terms “endothermic” and “exothermic” are relative. A reaction that is endothermic in one context may be exothermic in another context, depending on the system and surroundings. Additionally, the energy changes associated with endothermic and exothermic reactions can be measured using thermodynamic principles and are typically represented by values such as the enthalpy change (ΔH) or the heat of reaction.
Definition and examples of endothermic reactions
Endothermic reactions are chemical reactions that absorb heat energy from their surroundings, resulting in a decrease in temperature. These reactions require an input of energy to proceed, usually in the form of heat. The energy is absorbed by the reactants, causing them to undergo a chemical change and form new products.
An endothermic reaction can be represented by the equation: Reactants + Heat Energy → Products.
One example of an endothermic reaction is the reaction between baking soda (sodium bicarbonate) and vinegar (acetic acid) to produce carbon dioxide gas, water, and sodium acetate. When the two substances are mixed, they react together by absorbing heat energy from the surroundings. This causes the reaction mixture to feel cold to the touch, as it is drawing heat away from the skin. The formation of carbon dioxide gas bubbles is also an indicator of an endothermic reaction.
Another example is the process of ice melting. When solid ice is exposed to a higher temperature, such as room temperature, it absorbs heat energy from the surroundings. This energy is used to break the bonds between the water molecules in the ice, converting it into liquid water. The process of melting ice is endothermic because it requires the input of heat energy.
Endothermic reactions are an important concept in chemistry as they play a role in many natural and industrial processes. Understanding these reactions helps scientists and engineers design and optimize reactions and processes for various applications.
Definition and examples of exothermic reactions
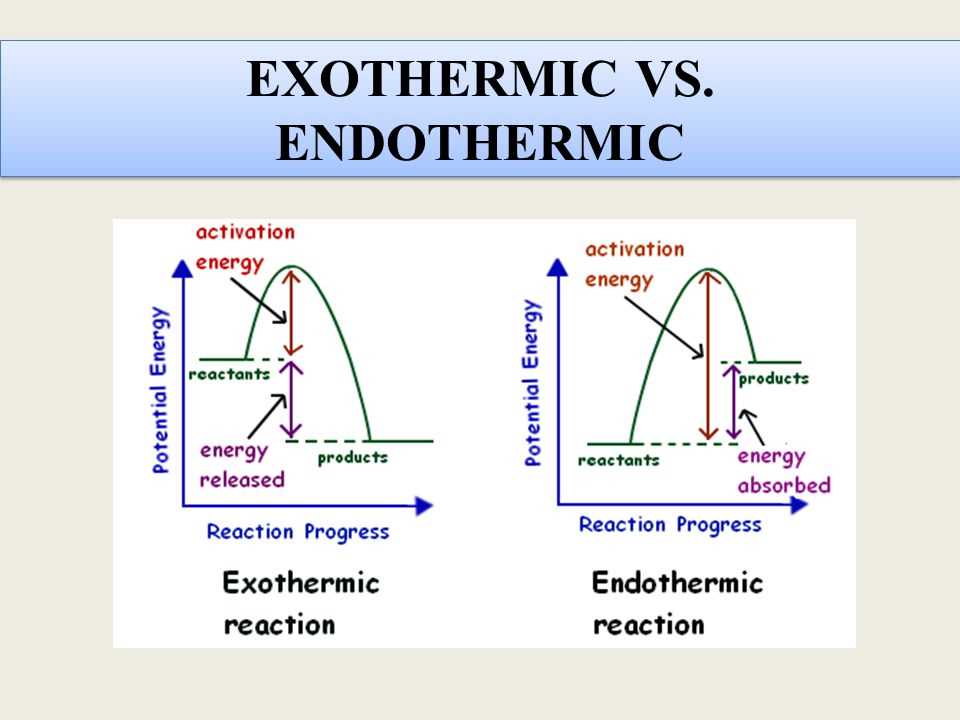
An exothermic reaction is a chemical reaction that releases energy in the form of heat or light. It is characterized by a decrease in the enthalpy of the system, meaning that the products of the reaction have a lower energy state than the reactants. Exothermic reactions occur spontaneously and often release a significant amount of energy.
One example of an exothermic reaction is the combustion of fuel, such as gasoline. When fuel reacts with oxygen in the presence of a spark or heat, it releases energy in the form of heat and light. This is why flames are produced during combustion. Another example of an exothermic reaction is the reaction between sodium hydroxide and hydrochloric acid, which produces salt and water while releasing heat.
In addition to combustion and neutralization reactions, exothermic reactions can also include processes such as oxidation and certain types of chemical bonding. For example, when iron reacts with oxygen to form iron oxide (rust), heat is released. Similarly, the reaction between hydrogen and chlorine to form hydrogen chloride is exothermic.
Exothermic reactions play a crucial role in various natural and industrial processes. They are used in the production of energy, such as in power plants and combustion engines. They are also involved in the release of light in fireworks and the generation of heat in cooking and heating appliances.
Examples of exothermic reactions:
- Combustion of fuel
- Neutralization reactions
- Oxidation reactions
- Chemical bonding reactions
Main differences between endothermic and exothermic reactions
Endothermic and exothermic reactions are two types of chemical reactions that differ in terms of heat exchange with the surroundings. In an endothermic reaction, heat is absorbed from the surroundings, while in an exothermic reaction, heat is released. This key difference in heat exchange leads to various contrasting characteristics between the two types of reactions.
Energy change: The main difference between endothermic and exothermic reactions lies in the energy change that occurs. In an endothermic reaction, the overall energy of the system increases as heat is absorbed. On the other hand, in an exothermic reaction, the overall energy of the system decreases as heat is released.
Temperature change: As a result of the energy change, endothermic and exothermic reactions also have different effects on temperature. In an endothermic reaction, the temperature of the surroundings decreases because heat is being absorbed from them. In contrast, in an exothermic reaction, the temperature of the surroundings increases due to the release of heat.
Reaction progress: Another difference between endothermic and exothermic reactions is the effect on the reaction progress. In an endothermic reaction, the reaction generally proceeds more slowly as heat is absorbed, as the reactants need to absorb energy to reach the activation energy threshold. In contrast, in an exothermic reaction, the release of heat provides the necessary energy for the reaction to proceed more rapidly.
Examples: Examples of endothermic reactions include the process of photosynthesis, where plants absorb sunlight to convert carbon dioxide and water into glucose and oxygen. Examples of exothermic reactions include combustion, where the burning of fuel releases heat energy.
Overall, the main differences between endothermic and exothermic reactions can be summarized by their respective energy changes, effects on temperature, reaction progress, and examples. Understanding these differences is crucial for understanding and predicting the behavior of different chemical reactions.
Heat transfer in endothermic reactions
In an endothermic reaction, heat transfer occurs from the surroundings into the system. This means that the system absorbs heat, resulting in a decrease in temperature of the surroundings. The heat transfer in endothermic reactions is an important factor to understand the energy changes that occur during the reaction.
During an endothermic reaction, energy is required to break the bonds in the reactants. This energy is absorbed from the surroundings in the form of heat. As a result, the surroundings cool down because they lose heat to the system. This heat transfer can be visualized as the reactants “sucking” in heat from their surroundings to fuel the reaction.
One common example of an endothermic reaction is the process of photosynthesis in plants. In this reaction, carbon dioxide and water are converted into glucose and oxygen. The energy needed for this reaction is absorbed from sunlight. The heat transfer from the sunlight to the plants allows them to convert light energy into chemical energy.
Overall, understanding the heat transfer in endothermic reactions is crucial in various fields, including chemistry, biology, and environmental science. By studying these reactions, scientists can better understand energy changes and develop more efficient processes for various applications.
Heat transfer in exothermic reactions
In exothermic reactions, heat is released or transferred from the system to the surroundings. This heat transfer typically occurs in three ways: conduction, convection, and radiation.
Conduction is the transfer of heat through direct contact between particles. In the context of exothermic reactions, this means that the high-energy particles involved in the reaction transfer heat to neighboring particles, causing them to increase in temperature. This process continues, resulting in the diffusion of heat throughout the system and eventually to the surroundings.
A second method of heat transfer is convection. Convection involves the movement of heat through the motion of fluids, such as gases or liquids. In exothermic reactions, convection occurs as the heated particles rise and are replaced by cooler particles, creating a flow of heat within the system. This heat is then carried away from the reaction site and into the surroundings.
The third method of heat transfer in exothermic reactions is radiation. Radiation involves the emission of electromagnetic waves, such as infrared radiation, which carry energy in the form of heat. In exothermic reactions, this radiation is emitted as a result of the high-energy particles involved in the reaction. The emitted radiation then travels through space and can be absorbed by the surroundings, transferring the heat energy away from the system.
In summary, in exothermic reactions, heat is transferred from the system to the surroundings through conduction, convection, and radiation. These processes allow the released heat energy to be distributed and carried away, resulting in an increase in temperature in the surroundings. Understanding these heat transfer mechanisms is important in the study and application of exothermic reactions in various fields, such as chemistry and thermodynamics.
Factors influencing the direction of endothermic and exothermic reactions

Endothermic and exothermic reactions are influenced by several factors that can determine the direction in which they proceed. These factors include temperature, concentration, pressure, and the presence of catalysts. Understanding these factors is crucial in predicting and controlling the outcome of chemical reactions.
Temperature plays a significant role in determining the direction of a reaction. In an exothermic reaction, increasing the temperature generally favors the formation of products, as it provides the necessary energy for the reaction to occur. On the other hand, in an endothermic reaction, increasing the temperature usually promotes the formation of reactants, as it allows for the absorption of heat energy from the surroundings.
The concentration of reactants also influences the direction of a reaction. In general, an increase in the concentration of reactants favors the formation of products in both endothermic and exothermic reactions. This is because a higher concentration provides more particles of reactants, leading to an increased frequency of successful collisions and, therefore, a higher likelihood of reaction.
Pressure can affect the direction of a reaction, particularly in gaseous systems. According to Le Chatelier’s principle, if a gaseous reaction involves a change in the number of moles of gas, then an increase in pressure will shift the equilibrium towards the side with fewer moles of gas. This principle applies to both endothermic and exothermic reactions and can be used to manipulate the equilibrium position.
Catalysts are substances that can speed up the rate of a reaction without being consumed in the process. They can also influence the direction of a reaction. Catalysts provide an alternative pathway for the reaction that has a lower activation energy. This allows the reaction to proceed more easily and may favor the formation of products in both endothermic and exothermic reactions.
Influence of temperature
Temperature plays a significant role in determining whether a reaction is endothermic or exothermic. In an endothermic reaction, the temperature of the surroundings decreases as energy is absorbed from the surroundings to fuel the reaction. On the other hand, in an exothermic reaction, the temperature of the surroundings increases as energy is released during the reaction.
The influence of temperature on the direction and rate of a chemical reaction can be explained by the principle of Le Chatelier. According to this principle, an increase in temperature favors the endothermic reaction, while a decrease in temperature favors the exothermic reaction. This means that raising the temperature can shift the equilibrium of a reaction towards the reactants, while lowering the temperature can shift the equilibrium towards the products.
Effects of temperature on reaction rate
The rate at which a chemical reaction occurs is also greatly influenced by temperature. In general, an increase in temperature leads to an increase in the rate of reaction, while a decrease in temperature leads to a decrease in the rate. This is because an increase in temperature results in an increase in the average kinetic energy of the reactant molecules, which in turn leads to more frequent and energetic collisions between the reactant molecules. These collisions are necessary for the reaction to occur. On the other hand, a decrease in temperature decreases the average kinetic energy of the molecules, resulting in fewer collisions and a slower reaction rate.
The influence of temperature on the rate of a reaction can be quantitatively described by the Arrhenius equation, which states that the rate constant of a reaction exponentially increases with an increase in temperature. This relationship emphasizes the direct relationship between temperature and reaction rate.