Energy diagrams are an essential tool in studying the behavior and transformations of energy in different systems. They provide a visual representation of how energy is transferred and transformed during a process. By understanding energy diagrams, we can gain insights into the factors that influence energy changes and the pathways through which energy flows.
In this article, we will explore the answers to various questions related to energy diagrams. We will discuss the different components of an energy diagram, such as the reactants, products, intermediates, and transition states. Additionally, we will delve into the concept of energy barriers and how they influence reaction rates.
Furthermore, we will examine the different types of energy diagrams, such as exothermic and endothermic reactions. We will explain how to interpret energy diagrams by identifying the energy changes, activation energy, and potential energy profiles. By providing detailed explanations and clear examples, this article aims to enhance your understanding of energy diagrams and their significance in the study of energy transformations.
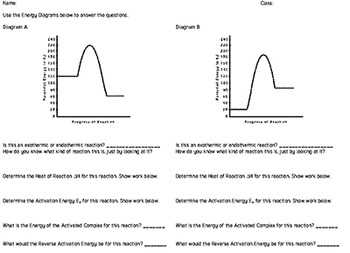
Energy Diagram Worksheet Answers
The energy diagram worksheet provides a visual representation of the energy changes that occur during a chemical or physical process. By analyzing the diagram, you can determine the energy of reactants, products, and intermediates, as well as identify the activation energy required for the reaction to occur.
When working on the energy diagram worksheet, you may be asked to identify the energy changes associated with different steps of a reaction, such as bond breaking and bond formation. Additionally, you may need to calculate the overall energy change for the reaction by comparing the energy of the reactants and the products.
Sample Answers:
- Step 1: The reactants start with a relatively high energy, indicating that energy is required for the reaction to occur. This energy is known as the activation energy.
- Step 2: During the reaction, the bonds of the reactants are broken, requiring energy input. This leads to an increase in energy.
- Step 3: After the bonds are broken, new bonds are formed between the atoms to create the products. This bond formation releases energy, leading to a decrease in energy.
- Step 4: The products have a lower energy compared to the reactants, indicating that energy is released during the reaction.
By analyzing the energy diagram worksheet, you can gain a better understanding of the energy changes that occur during a chemical process. This knowledge is crucial for predicting and understanding the outcome of reactions, as well as for optimizing reaction conditions.
Understanding Potential Energy
Potential energy is a fundamental concept in the study of energy and its transformations. It refers to the energy that an object possesses due to its position, shape, or composition. Unlike kinetic energy, which is the energy of motion, potential energy is a type of stored energy that has the potential to be converted into other forms of energy.
Types of potential energy:
- Gravitational potential energy: This type of potential energy is associated with an object’s position above the Earth’s surface. The higher an object is lifted, the greater its gravitational potential energy.
- Elastic potential energy: Elastic potential energy is the energy stored in elastic materials, such as a stretched rubber band or a compressed spring. As these materials are deformed, they possess the potential to return to their original shape and release the stored energy.
- Chemical potential energy: Chemical potential energy is stored in the bonds between atoms and molecules. When chemical reactions occur, these bonds can be broken, releasing the stored energy.
- Nuclear potential energy: This type of potential energy is associated with the strong nuclear force that holds atomic nuclei together. During nuclear reactions, such as fission or fusion, this energy is released.
Potential energy can be converted into kinetic energy when an object is set in motion. For example, when a ball is lifted and then dropped, its gravitational potential energy is converted into kinetic energy as it falls. Similarly, when a compressed spring is released, its elastic potential energy is transformed into kinetic energy as the spring expands.
Understanding potential energy is essential in various fields, including physics, engineering, and environmental science. It helps explain the behavior of objects and can be used to analyze and design systems that involve energy transformations. By understanding the different types of potential energy and how they can be harnessed, we can develop more efficient and sustainable energy sources.
Interpreting Energy Diagrams
Energy diagrams are graphical representations that show the energy changes that occur during a chemical reaction or a physical process. They provide valuable information about the reaction’s energy profile, including the activation energy, the overall energy change, and the stability of the reactants and products.
When interpreting energy diagrams, it is important to understand the key components and their significance. The horizontal axis represents the progress of the reaction, while the vertical axis represents the energy of the system. The reactants are shown on the left side of the diagram, and the products are shown on the right side.
The first important feature to consider is the energy difference between the reactants and the products. If the products have a lower energy than the reactants, the reaction is exothermic, meaning it releases energy. On the other hand, if the products have a higher energy than the reactants, the reaction is endothermic, meaning it absorbs energy.
Another important feature is the activation energy, which is the energy barrier that the reactants must overcome to form the products. It is represented by the highest point on the diagram, called the transition state or the activated complex. The lower the activation energy, the faster the reaction will occur.
Energy diagrams can also show the presence of catalysts, which are substances that increase the reaction rate without being consumed in the process. Catalysts lower the activation energy and provide an alternative reaction pathway, as shown by a separate line on the diagram.
Overall, energy diagrams are powerful tools for understanding and analyzing the energy changes associated with chemical reactions and physical processes. By studying these diagrams, scientists can gain insights into the thermodynamics and kinetics of a reaction, which can be useful in various fields, including chemistry, biology, and engineering.
Activation Energy
The activation energy is a key concept in understanding the kinetics of chemical reactions. It represents the minimum energy required for a reaction to occur. In other words, it is the energy barrier that must be overcome for reactant molecules to transform into product molecules. The activation energy can be thought of as the “push” that is needed to start a reaction.
The activation energy can be visualized using an energy diagram. In an energy diagram, the reactants are placed at a higher energy level than the products. The activation energy is then represented as the difference in energy between the reactants and the highest point on the diagram, known as the transition state or activated complex.
The activation energy depends on several factors, including the nature of the reactants, the temperature, and the presence of a catalyst. Generally, reactions with higher activation energies proceed more slowly than reactions with lower activation energies. This is because higher activation energies require a greater amount of thermal energy to be supplied to the reactants in order for them to overcome the energy barrier and react.
Catalysts can lower the activation energy by providing an alternate reaction pathway with a lower energy barrier. They do this by temporarily binding to the reactant molecules and altering their geometries or electronic structures, making it easier for the reaction to occur. As a result, catalysts can significantly increase the rate of a reaction by lowering the activation energy.
Exothermic and Endothermic reactions
Chemical reactions can be classified into two types based on the energy changes that occur during the reaction: exothermic reactions and endothermic reactions. These terms describe the direction of heat flow during the reaction.
In an exothermic reaction, heat is released as a byproduct. This means that the energy of the reactants is higher than the energy of the products. The overall energy change is negative, indicating the release of energy. Exothermic reactions are often accompanied by an increase in temperature, as the heat is transferred to the surrounding environment. Examples of exothermic reactions include combustion reactions, such as the burning of fuels, and neutralization reactions, such as the reaction between an acid and a base.
On the other hand, in an endothermic reaction, heat is absorbed from the surroundings. This means that the energy of the products is higher than the energy of the reactants. The overall energy change is positive, indicating the absorption of energy. Endothermic reactions often result in a decrease in temperature, as the heat is taken in from the surrounding environment. Examples of endothermic reactions include photosynthesis, where plants absorb energy from sunlight to convert carbon dioxide and water into glucose, and the melting of ice, where heat is required to convert the solid ice into liquid water.
Understanding whether a reaction is exothermic or endothermic is important in various applications. In industries, the knowledge of exothermic reactions is used to design and optimize processes that generate heat, such as in the production of energy from fuels. On the other hand, endothermic reactions are utilized in processes that require cooling, such as in refrigeration or air conditioning systems. Overall, the classification of reactions into exothermic and endothermic provides valuable insight into the energy changes that occur during chemical reactions and their implications in various fields.
Factors Affecting Energy Diagrams
In chemistry, energy diagrams are graphical representations used to visualize the energy changes that occur during a chemical reaction. These diagrams show the energy levels of the reactants, intermediates, and products, as well as the activation energy required for the reaction to take place. Several factors can influence the shape and features of energy diagrams.
1. Nature of the reactants and products: Different chemical reactions involve different reactants and products, which can have varying energy levels. The nature of the atoms and molecules involved can affect the stability and energy of the compounds, leading to differences in the energy diagram. Reactants or products with higher energy levels may require more or less activation energy for the reaction to occur.
2. Reaction mechanism: The pathway or mechanism by which a reaction takes place can also have an impact on the energy diagram. Complex reactions often involve multiple steps with different activation energies. Each step contributes to the overall energy diagram, and the rate-determining step is usually the one with the highest activation energy.
3. Temperature and pressure: Changes in temperature and pressure can affect the energy diagram by influencing the energy levels of the reactants, products, and intermediates. Increasing temperature generally leads to higher energy levels and lower activation energy, while high pressure can aid in the formation of transition states and lower the activation energy barrier.
4. Catalysts: Catalysts are substances that can speed up a chemical reaction by providing an alternative pathway with lower activation energy. The presence of a catalyst can change the energy diagram by reducing the activation energy required for the reaction to occur. This allows the reaction to happen more quickly and efficiently.
5. Concentration and environment: The concentration of reactants can also influence the energy diagram. Higher concentrations can lead to a higher probability of reactant collisions and potentially lower the activation energy barrier. Additionally, the environment in which the reaction takes place, such as the presence of a solvent or other molecules, can affect the energy levels and stability of the compounds involved.
In conclusion, energy diagrams are influenced by several factors, including the nature of the reactants and products, the reaction mechanism, temperature and pressure, the presence of catalysts, and the concentration and environment. Understanding these factors is crucial for predicting and analyzing the energy changes in chemical reactions.
Energy Diagram Worksheet Examples
Energy diagrams are a helpful tool used in chemistry to visualize and understand the energy changes that occur during a chemical reaction. These diagrams show the energy levels of the reactants, products, and intermediates, as well as the activation energy and the overall energy change of the reaction. Looking at energy diagrams can provide valuable information about the reaction kinetics and the stability of the different species involved.
One example of an energy diagram worksheet might involve a simple exothermic reaction, such as the combustion of methane. The energy diagram would show the reactants, methane and oxygen, at a higher energy level than the products, carbon dioxide and water. The energy difference between the reactants and products represents the energy released during the reaction. The activation energy, which is the energy required to start the reaction, would also be shown on the diagram.
Energy Diagram Worksheet Example 1:
- Reactants: Methane and oxygen
- Products: Carbon dioxide and water
- Activation energy: 50 kJ/mol
- Overall energy change: -800 kJ/mol
Another example of an energy diagram worksheet could involve an endothermic reaction, such as the decomposition of calcium carbonate. In this reaction, the energy diagram would show the reactant, calcium carbonate, at a higher energy level than the products, calcium oxide and carbon dioxide. The energy difference between the reactant and products represents the energy absorbed during the reaction. The activation energy and overall energy change would also be indicated on the diagram.
Energy Diagram Worksheet Example 2:

- Reactant: Calcium carbonate
- Products: Calcium oxide and carbon dioxide
- Activation energy: 100 kJ/mol
- Overall energy change: +500 kJ/mol
These examples demonstrate how energy diagrams can be used to represent different types of reactions and provide information about the energy changes involved. By analyzing energy diagrams, chemists can gain insight into the thermodynamics and kinetics of chemical reactions, which can be used to optimize reaction conditions, design catalysts, and understand the feasibility of reactions.