Energy plays a fundamental role in chemical reactions, influencing the outcome of these processes and driving the transformation of substances. In the study of chemistry, it is crucial to understand the concepts and principles related to energy and how it is involved in chemical reactions.
Chemical reactions involve the breaking and forming of chemical bonds, which requires energy. The amount of energy required to break or form bonds is known as the energy of activation. This energy can come from various sources, such as heat or light, and is necessary to initiate a reaction.
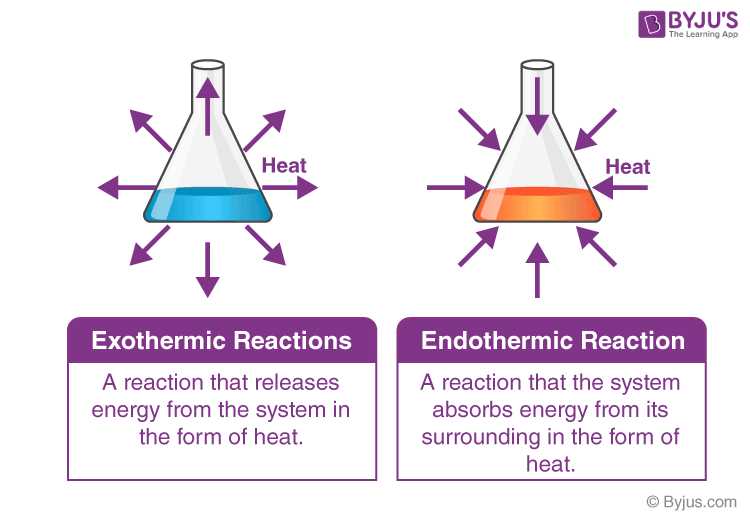
During a chemical reaction, energy can be released or absorbed in the form of heat. Exothermic reactions release energy, usually in the form of heat, into the surrounding environment. These reactions often result in the production of heat, light, or sound, and can be observed as a temperature increase. On the other hand, endothermic reactions absorb energy from the surroundings, resulting in a decrease in temperature.
The conservation of energy is a vital principle in chemistry. According to the law of conservation of energy, energy is neither created nor destroyed; it simply changes form. This principle applies to chemical reactions as well. The total energy of the reactants before a reaction is equal to the total energy of the products after the reaction. This understanding allows chemists to predict the energy changes that occur during a reaction and analyze the effects of energy on the overall process.
What is Energy in Chemical Reactions?
In chemical reactions, energy plays a crucial role in determining the outcome and feasibility of the reaction. Energy is defined as the capacity to do work or supply heat, and it exists in various forms, including kinetic energy, potential energy, and thermal energy. In the context of chemical reactions, energy is either absorbed or released during the process.
Different types of energy changes occur in chemical reactions. One of the most common types is the exchange of kinetic energy, which is the energy associated with the movement of particles. When reactant molecules collide, they transfer kinetic energy to each other, leading to the breaking and forming of chemical bonds.
Another important form of energy change in chemical reactions is the exchange of potential energy. Potential energy is the energy stored in chemical bonds, and it determines the stability of the molecules involved. Breaking bonds requires energy input, while forming bonds releases energy. This exchange of potential energy drives the rearrangement of atoms in a reaction.
Additionally, heat energy is often involved in chemical reactions. Reactions can be exothermic, where heat is released to the surroundings, or endothermic, where heat is absorbed from the surroundings. The amount of heat released or absorbed during a reaction is known as the enthalpy change and can be measured experimentally.
In conclusion, energy is a fundamental concept in chemical reactions. It is exchanged in various forms, including kinetic energy, potential energy, and heat energy. Understanding and controlling energy changes in reactions is essential for predicting and manipulating chemical processes.
Importance of Understanding Energy in Chemical Reactions
Energy is a fundamental concept in chemistry, and understanding its role in chemical reactions is crucial for a comprehensive understanding of the subject.
Chemical reactions involve the transformation of one set of substances, called reactants, into another set of substances, known as products. These transformations are driven by the change in energy that occurs during the reaction. Energy can be neither created nor destroyed; instead, it is transferred or transformed from one form to another. Therefore, an understanding of the energy changes that occur during chemical reactions is essential for predicting the outcome of a reaction.
One of the key energy concepts in chemical reactions is the concept of activation energy. Activation energy is the minimum amount of energy that must be supplied to initiate a chemical reaction. It is often represented by the energy barrier that must be overcome for the reaction to occur. By understanding the concept of activation energy, chemists can design and optimize reaction conditions to increase the reaction rate and yield.
Furthermore, understanding the energy changes associated with chemical reactions allows chemists to determine the energy efficiency of a reaction. Energy efficiency refers to the amount of energy that is converted into useful work or products compared to the total energy input. By maximizing energy efficiency, chemists can develop more sustainable and environmentally friendly processes.
In conclusion, a thorough understanding of energy in chemical reactions is vital for predicting reaction outcomes, optimizing reaction conditions, and developing sustainable processes. By grasping the concepts of energy changes, activation energy, and energy efficiency, chemists can make significant strides in advancing the field of chemistry and contributing to the development of new technologies.
Efficient use of resources
In today’s world, efficient use of resources has become a critical issue due to the increasing demand for energy and the environmental concerns associated with it. The efficient use of resources refers to maximizing the utility or output derived from a given set of resources while minimizing waste and negative environmental impacts.
One way to achieve efficient use of resources is through the adoption of energy-saving technologies and practices. These technologies aim to reduce energy consumption by improving the energy efficiency of various processes and systems. For example, the use of energy-efficient appliances, lighting systems, and insulation materials can significantly reduce energy consumption in households and commercial buildings.
Another aspect of resource efficiency is the optimization of manufacturing processes and supply chains. By adopting lean production techniques and implementing resource-efficient manufacturing strategies, companies can reduce wastage and improve productivity. This includes measures such as recycling and reusing materials, reducing packaging waste, and optimizing transportation routes to minimize fuel consumption.
An important component of resource efficiency is the promotion of sustainable practices and renewable energy sources. By shifting towards renewable energy sources like solar, wind, and hydroelectric power, we can reduce our reliance on fossil fuels and mitigate the negative environmental impacts associated with their extraction and combustion. Additionally, embracing sustainable practices such as water conservation and responsible waste management can contribute to the efficient use of resources.
In conclusion, efficient use of resources is crucial for addressing the challenges related to energy demand and environmental sustainability. By adopting energy-saving technologies, optimizing manufacturing processes, and promoting renewable energy sources, we can ensure the responsible and sustainable use of resources for a better future.
Predicting reaction outcomes
When studying chemical reactions, it is often useful to be able to predict the possible outcomes of a reaction based on the reactants and the conditions under which the reaction is carried out. This can help chemists understand and control reactions, as well as design new processes and materials.
One important factor to consider when predicting reaction outcomes is the energy change that occurs during the reaction. The energy change can be either endothermic or exothermic, depending on whether the reaction absorbs or releases energy. This can be determined by comparing the energy of the reactants to the energy of the products.
In addition to energy changes, reaction outcomes can also be influenced by factors such as the presence of catalysts, temperature, and pressure. Catalysts can speed up reactions by lowering the activation energy, while temperature and pressure can affect the rate and direction of a reaction. By considering all these factors, chemists can make predictions about the likely products of a reaction.
However, it is important to note that predicting reaction outcomes is not always straightforward. Chemical reactions can be complex, and there may be multiple possible products depending on the conditions. Additionally, unexpected side reactions or competing reactions can occur, leading to different outcomes. Therefore, experimental verification is often necessary to confirm predictions and fully understand the reaction.
Designing and optimizing chemical processes
Designing and optimizing chemical processes is a complex and crucial task in the field of chemistry. It involves the development and improvement of processes for the production of chemicals and their derivatives, with the aim of achieving higher efficiency, sustainability, and profitability. The design process requires a thorough understanding of the desired product, the raw materials, and the reaction kinetics.
One key aspect of designing chemical processes is the selection of catalysts. Catalysts are essential in speeding up chemical reactions and reducing energy requirements. They play a vital role in determining the efficiency and selectivity of the process. Through careful selection and optimization of catalysts, scientists and engineers can enhance the reaction rates, achieve higher yields, and minimize the formation of unwanted by-products.
The optimization of chemical processes also involves the consideration of various parameters, such as temperature, pressure, and the concentration of reactants. These factors affect the reaction kinetics and the overall energy requirements of the process. By carefully controlling and optimizing these parameters, scientists can minimize energy consumption, reduce waste, and improve the overall sustainability of the process.
In addition to catalyst selection and parameter optimization, the design of chemical processes also involves the consideration of process safety and environmental impact. Engineers need to ensure that the process is not only efficient and profitable but also safe for both operators and the environment. This includes the implementation of proper safety protocols, the reduction of hazardous waste, and the use of environmentally friendly solvents and raw materials.
Overall, designing and optimizing chemical processes requires a multidisciplinary approach, involving the collaboration of chemists, engineers, and environmental scientists. Through careful consideration of catalysts, reaction parameters, safety measures, and environmental impact, scientists and engineers can develop efficient, sustainable, and economically viable chemical processes.
Types of Energy in Chemical Reactions
Chemical reactions involve the transformation of one or more substances into new substances. This transformation is accompanied by a transfer of energy. In chemical reactions, there are several types of energy that can be involved:
- Heat Energy: Heat energy is the most common form of energy involved in chemical reactions. It is often supplied to the reaction from an external source, such as a burner or heater. Heat energy can be used to break the bonds between atoms in the reactant molecules, allowing new bonds to form in the product molecules.
- Light Energy: Light energy can be involved in certain chemical reactions, especially those that are photochemical reactions. These reactions are initiated or promoted by the absorption of light energy. The absorbed light energy can excite electrons, leading to the formation of new chemical species.
- Electrical Energy: Electrical energy can also be involved in chemical reactions. This is observed in electrolysis reactions, where an electric current is passed through a solution to drive a chemical reaction. The electrical energy is used to break down the reactant molecules into ions, allowing new compounds to form.
- Potential Energy: Potential energy is associated with the position or arrangement of particles in a chemical reaction. It is related to the energy stored in chemical bonds, and changes in potential energy occur as bonds are broken and new bonds are formed. Chemical reactions that release energy result in a decrease in potential energy, while reactions that absorb energy result in an increase in potential energy.
These different types of energy play important roles in chemical reactions and are essential for driving the transformation of reactants into products. Understanding the types of energy involved can help scientists design and control chemical reactions for various applications in fields such as energy production, pharmaceuticals, and materials science.
Potential energy
Potential energy is a form of energy that an object possesses due to its position or condition. It is the energy that is stored and waiting to be released. Objects have potential energy because of their composition or structure. This energy can be converted into other forms, such as kinetic energy, which is the energy of motion.
There are several types of potential energy, including gravitational potential energy, elastic potential energy, and chemical potential energy. Gravitational potential energy is the energy that an object possesses due to its position in a gravitational field. It is determined by the height and weight of the object. Elastic potential energy is the energy stored in an elastic object, such as a stretched spring or a compressed rubber band. Chemical potential energy is the energy stored in the bonds of chemical compounds. It is released when a chemical reaction occurs.
Potential energy can be calculated using specific formulas depending on the type of energy involved. For example, the formula for gravitational potential energy is PE = mgh, where PE is the potential energy, m is the mass of the object, g is the acceleration due to gravity, and h is the height of the object. Elastic potential energy can be calculated using the formula PE = 0.5kx^2, where PE is the potential energy, k is the spring constant, and x is the displacement from the equilibrium position. Chemical potential energy is more complex and depends on the specific compounds involved in the reaction.
Understanding potential energy is important in the study of chemical reactions as it helps explain why reactions occur and how energy is transferred. It also plays a crucial role in various fields such as physics, engineering, and environmental science. By harnessing and manipulating potential energy, scientists and engineers can develop innovative technologies and systems that improve our lives and address global challenges.