
Chemical reactions are fundamental processes that occur in various natural and man-made systems. Understanding the energy changes that accompany these reactions is crucial for studying and predicting their outcomes. The Energy in Chemical Reactions Unit Test is designed to assess students’ knowledge and understanding of the concepts and principles related to energy changes in chemical reactions.
The test covers topics such as exothermic and endothermic reactions, heat transfer, activation energy, and the role of catalysts. Students will be required to analyze reaction equations, calculate and interpret enthalpy changes, and identify the factors that influence the energy change in a reaction.
By testing students’ understanding of energy in chemical reactions, this unit test aims to evaluate their ability to apply the principles of thermodynamics to real-world scenarios. It also serves as a means for teachers to gauge their students’ comprehension of this important topic and identify areas that may require further instruction or clarification.
Energy in Chemical Reactions Unit Test
In the study of chemistry, one important aspect is understanding how energy is involved in chemical reactions. This is the focus of the Energy in Chemical Reactions unit test. The test aims to assess students’ knowledge and understanding of concepts such as energy transfer, endothermic and exothermic reactions, activation energy, and factors affecting reaction rates.
The test may include multiple-choice questions, short answer questions, and problem-solving questions. Students will be required to apply their knowledge of energy changes in chemical reactions to solve problems, analyze experimental data, and interpret reaction diagrams. They should be able to identify the type of reaction as either endothermic or exothermic, and explain the energy changes that occur during the reaction.
Furthermore, students will be tested on their understanding of activation energy and how it affects reaction rates. They should be able to explain the concept of activation energy and how it relates to the energy required for a reaction to occur. They may also be asked to discuss factors that can affect reaction rates, such as temperature, concentration, and catalysts.
This unit test is designed to assess students’ comprehension of the energy changes that occur during chemical reactions and their ability to apply that knowledge to real-world situations. It is an important topic in chemistry as it plays a crucial role in understanding and predicting the behavior of chemical systems. By successfully completing this test, students will demonstrate their understanding of these concepts and their ability to apply them in various contexts.
Definition of Energy in Chemical Reactions
The concept of energy in chemical reactions refers to the ability of a system to do work or transfer heat. It is an essential component in understanding and analyzing chemical reactions, as energy changes play a crucial role in determining whether a reaction will occur and the extent to which it will proceed.
In a chemical reaction, energy can be transferred between the reactants and products, resulting in changes in their internal energy. These energy changes may occur in the form of heat, light, or mechanical work. The study of energy changes in chemical reactions falls under the branch of thermodynamics.
One of the fundamental principles governing energy in chemical reactions is the law of conservation of energy. According to this law, energy can neither be created nor destroyed in a chemical reaction, but it can be converted between different forms. This means that the total energy of the reactants must be equal to the total energy of the products.
During a chemical reaction, energy can be either released (exothermic reaction) or absorbed (endothermic reaction). In an exothermic reaction, the products have lower energy than the reactants, and the excess energy is released in the form of heat or light. On the other hand, in an endothermic reaction, the products have higher energy than the reactants, and energy is absorbed from the surroundings.
The concept of energy in chemical reactions is crucial in understanding and predicting the behavior of various chemical systems and reactions. It allows scientists to calculate and analyze energy changes, understand reaction kinetics, and design reactions with specific energy requirements. It also plays a significant role in various industries, such as the field of energy production and storage, where the efficient utilization and transformation of energy in chemical reactions are of great importance.
Overall, the definition of energy in chemical reactions encompasses the transfer and conversion of energy within a system, providing valuable insights into the behavior and dynamics of chemical systems.
Types of Energy Involved in Chemical Reactions
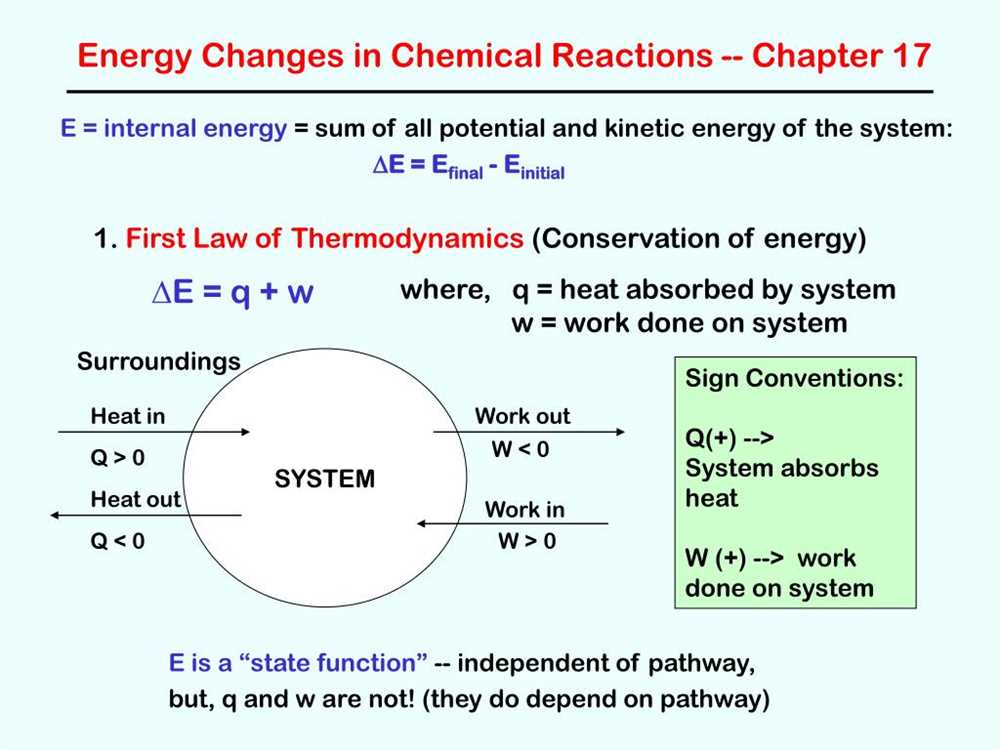
In chemical reactions, energy is involved in various forms, playing a crucial role in driving and sustaining the process. These different types of energy can be categorized into two main groups: potential energy and kinetic energy.
Potential Energy: Potential energy refers to the stored energy that a substance possesses based on its position or composition. In chemical reactions, potential energy can exist in several forms such as chemical potential energy, electrical potential energy, and gravitational potential energy.
Chemical potential energy: Chemical potential energy is the energy stored in the chemical bonds of a substance. When a chemical reaction occurs, the bonds between atoms are either broken or formed, resulting in a release or absorption of energy. For example, in exothermic reactions, the reactants have higher chemical potential energy than the products, leading to the release of heat. On the other hand, endothermic reactions absorb energy, resulting in products with higher chemical potential energy.
Electrical potential energy: Electrical potential energy is the energy associated with the interaction of charged particles. This type of potential energy is often involved in reactions that occur in electrolytic cells or involve the transfer of electrons between species.
Gravitational potential energy: Gravitational potential energy is the energy an object possesses due to its position relative to a gravitational field. While not as commonly involved in chemical reactions, gravitational potential energy may play a role in some reactions involving substances at different heights or positions in a system.
Kinetic Energy: Kinetic energy refers to the energy of motion. In chemical reactions, it primarily manifests in the form of thermal energy, which is associated with the random motion of particles.
As the reactants collide and interact, their kinetic energy increases, leading to an increase in their temperature. The kinetic energy of particles determines the rate at which a reaction occurs, as higher kinetic energy increases the likelihood of effective molecular collisions.
Relationship Between Energy and Chemical Reactions
The relationship between energy and chemical reactions is essential in understanding how reactions occur and the changes in energy that accompany them. In chemical reactions, energy is either released or absorbed as the bonds between atoms are broken and formed. This transfer of energy is dependent on the difference in potential energy between the reactants and the products.
Activation energy: Every chemical reaction requires a certain amount of energy to initiate the reaction, known as activation energy. This energy is needed to break the existing bonds in the reactants and allow new bonds to form in the products. Activation energy acts as a barrier that must be overcome for the reaction to proceed.
Exothermic reactions: In exothermic reactions, the energy released during the reaction is greater than the energy required to break the bonds in the reactants. As a result, the overall energy change is negative, and the surroundings gain energy. Examples of exothermic reactions include combustion and certain types of oxidation reactions.
Endothermic reactions: In endothermic reactions, the energy required to break the bonds in the reactants is greater than the energy released during the formation of new bonds in the products. As a result, the overall energy change is positive, and the surroundings lose energy. Examples of endothermic reactions include photosynthesis and the dissociation of ammonium nitrate.
Energy diagrams: Energy diagrams, also known as potential energy diagrams, are graphical representations of the energy changes that occur during a chemical reaction. They show the activation energy, the energy of the reactants, and the energy of the products. By analyzing these diagrams, scientists can gain insight into the energy profile of a reaction and understand its feasibility and rate.
Factors affecting the energy of chemical reactions: The energy of chemical reactions can be influenced by various factors, including temperature, concentration, and catalysts. Increasing the temperature generally increases the rate of reactions by providing more energy to overcome the activation energy. Concentration affects reaction rates by increasing the likelihood of successful collisions between reacting particles. Catalysts, on the other hand, lower the activation energy required for a reaction, making it easier for the reactants to transform into products.
In conclusion

Understanding the relationship between energy and chemical reactions is crucial for predicting and controlling various chemical processes. Whether it’s the activation energy required for a reaction to start, the energy changes during an exothermic or endothermic reaction, or the factors that affect the energy of reactions, energy plays a fundamental role in determining the feasibility and speed of chemical reactions.
Activation Energy
In chemistry, activation energy refers to the energy required for a chemical reaction to occur. It is the minimum amount of energy needed for the reactant molecules to successfully overcome the energy barrier and transform into products. Activation energy can be thought of as the “hill” that the reactant molecules must climb in order to reach the other side and form products. It is often represented by the symbol Ea.
The concept of activation energy is important because it determines the rate at which a chemical reaction takes place. Higher activation energy barriers lead to slower reaction rates, while lower activation energy barriers result in faster reaction rates. The magnitude of the activation energy depends on several factors, such as the nature of the reactants, the temperature, and the presence of catalysts.
A catalyst is a substance that can lower the activation energy of a reaction, thus increasing the reaction rate. It does so by providing an alternative reaction pathway with a lower energy barrier. By lowering the activation energy, catalysts increase the number of reactant molecules that have enough energy to overcome the barrier, effectively speeding up the reaction.
Understanding activation energy is fundamental in the field of chemical kinetics, which studies the rates of chemical reactions. By studying the activation energy of a reaction, scientists can gather information about the reaction mechanism and make predictions about the reaction rate under different conditions. Activation energy is also important in industries such as pharmaceuticals, where the efficiency and speed of chemical reactions are crucial for the production of drugs.
Exothermic vs. Endothermic Reactions
Chemical reactions can be classified as either exothermic or endothermic based on the energy changes that occur during the reaction. In an exothermic reaction, energy is released to the surroundings in the form of heat. This means that the products of the reaction have less energy than the reactants. The release of energy in exothermic reactions can often be observed as heat, light, or sound. Examples of exothermic reactions include combustion reactions, such as burning wood or gasoline.
On the other hand, endothermic reactions absorb energy from the surroundings. In these reactions, the products have more energy than the reactants. This energy is typically obtained from the surrounding environment in the form of heat. As a result, endothermic reactions often feel cold to the touch. An example of an endothermic reaction is the process of photosynthesis, where plants absorb energy from sunlight to convert carbon dioxide and water into glucose and oxygen. Another example is the reaction between baking soda and vinegar, which requires an input of heat to occur.
Key Differences
- In exothermic reactions, energy is released to the surroundings, while in endothermic reactions, energy is absorbed from the surroundings.
- Exothermic reactions often give off heat, light, or sound, whereas endothermic reactions may feel cold to the touch.
- Examples of exothermic reactions include combustion reactions, while examples of endothermic reactions include photosynthesis and the reaction between baking soda and vinegar.
Understanding the difference between exothermic and endothermic reactions is crucial in various fields, such as chemistry and thermodynamics. It allows scientists to predict and control the energy changes that occur during chemical reactions, which is essential for the development of new materials, fuels, and processes.
Factors Affecting Energy Changes in Chemical Reactions
The energy changes that occur during chemical reactions are influenced by various factors. The main factors that affect these energy changes include the nature of the reactants and products, the concentration and pressure of the reactants, the temperature, and the presence of a catalyst. Each of these factors plays a significant role in determining the overall energy change of a reaction.
The nature of the reactants and products involved in a chemical reaction is an important factor in determining the energy change. Different types of chemical bonds, such as ionic and covalent bonds, require different amounts of energy to break and form. Therefore, reactions involving different types of reactants and products will have different energy changes. For example, a reaction between two highly reactive elements, such as sodium and chlorine, will release a large amount of energy due to the formation of strong ionic bonds in the product, sodium chloride.
- Concentration and pressure: The concentration and pressure of the reactants can also affect the energy change in a chemical reaction. An increase in concentration or pressure can increase the frequency of collisions between reactant particles, leading to more successful collisions and a higher energy change. For example, increasing the concentration of hydrogen and oxygen gases in a reaction to form water will increase the energy change due to the increased number of collisions and successful bond formations.
- Temperature: The temperature of a reaction is another important factor that affects energy changes. As the temperature increases, the particles of the reactants gain more kinetic energy, resulting in more frequent and energetic collisions. This leads to a higher energy change in the reaction. For example, heating a reaction between iron and sulfur will increase the energy change due to the increased kinetic energy of the reactant particles.
- Catalysts: Catalysts are substances that increase the rate of a chemical reaction without being permanently consumed. They can also affect the energy change of a reaction. Catalysts provide an alternative pathway for the reaction, reducing the activation energy required for the reaction to occur. This leads to a lower overall energy change in the reaction. For example, the addition of a catalyst such as platinum in the reaction between hydrogen and oxygen gases to form water will lower the energy change.
In conclusion, the energy changes in chemical reactions are influenced by various factors such as the nature of the reactants and products, the concentration and pressure of the reactants, the temperature, and the presence of a catalyst. Understanding these factors is crucial in predicting and controlling the energy changes that occur in chemical reactions.