Enthalpy is an important concept in thermodynamics that measures the heat energy in a system at constant pressure. It plays a crucial role in various scientific and engineering applications, including chemical reactions, phase transitions, and energy transfer.
To fully understand and apply the concept of enthalpy, it is important to practice solving problems. That’s why we have compiled a collection of enthalpy practice problems with answers in PDF format. These problems cover a wide range of scenarios and will help you develop a strong understanding of enthalpy calculations.
Each problem in the PDF includes a specific scenario and a set of given data, such as initial and final temperatures, pressure, and heat capacity. Your task is to determine the change in enthalpy (ΔH) using the appropriate equations and thermodynamic principles.
By solving these practice problems, you will gain valuable hands-on experience in applying enthalpy calculations to real-world situations. Whether you are a student studying thermodynamics or a professional in the field, these practice problems will enhance your problem-solving skills and deepen your understanding of enthalpy.
What is enthalpy and why is it important?
Enthalpy is a thermodynamic property of a system, represented by the symbol H. It is defined as the sum of the internal energy of the system plus the product of pressure and volume. Enthalpy can be thought of as a measure of the total energy content of a system, and it is commonly used to describe heat transfer during chemical reactions and physical processes.
Enthalpy is important because it allows us to understand and predict the heat changes that occur during various processes. By studying enthalpy changes, scientists and engineers can optimize chemical reactions, determine the feasibility of industrial processes, and design energy-efficient systems.
Enthalpy is particularly useful in the field of chemistry, where it helps to explain and quantify the heat changes that occur during reactions. The enthalpy change of a reaction, also known as the heat of reaction, can provide valuable information about the energy released or absorbed during a chemical reaction.
Enthalpy is also important in the field of thermodynamics, where it is used to analyze and design energy systems. By understanding the enthalpy changes associated with different processes, engineers can optimize energy efficiency and minimize energy losses.
Overall, enthalpy is a fundamental concept in thermodynamics and is essential for understanding and predicting heat transfer in chemical reactions and physical processes. Its importance extends to various scientific and engineering disciplines, making it a crucial concept to grasp.
How is enthalpy calculated?
Enthalpy is a thermodynamic quantity that measures the heat content of a system. It is denoted by the symbol “H” and is often expressed in units of joules (J) or kilojoules (kJ). Enthalpy can be calculated using various equations and principles from thermodynamics.
One way to calculate enthalpy is through the equation:
H = U + PV, where H is the enthalpy, U is the internal energy of the system, P is the pressure, and V is the volume of the system. This equation is based on the fact that enthalpy is the sum of the internal energy and the product of pressure and volume.
In some cases, the change in enthalpy (ΔH) can be calculated using the equation:
ΔH = ΔU + PΔV, where ΔH is the change in enthalpy, ΔU is the change in internal energy, P is the pressure, and ΔV is the change in volume. This equation is commonly used in thermodynamics to calculate the heat transfer in a system at constant pressure.
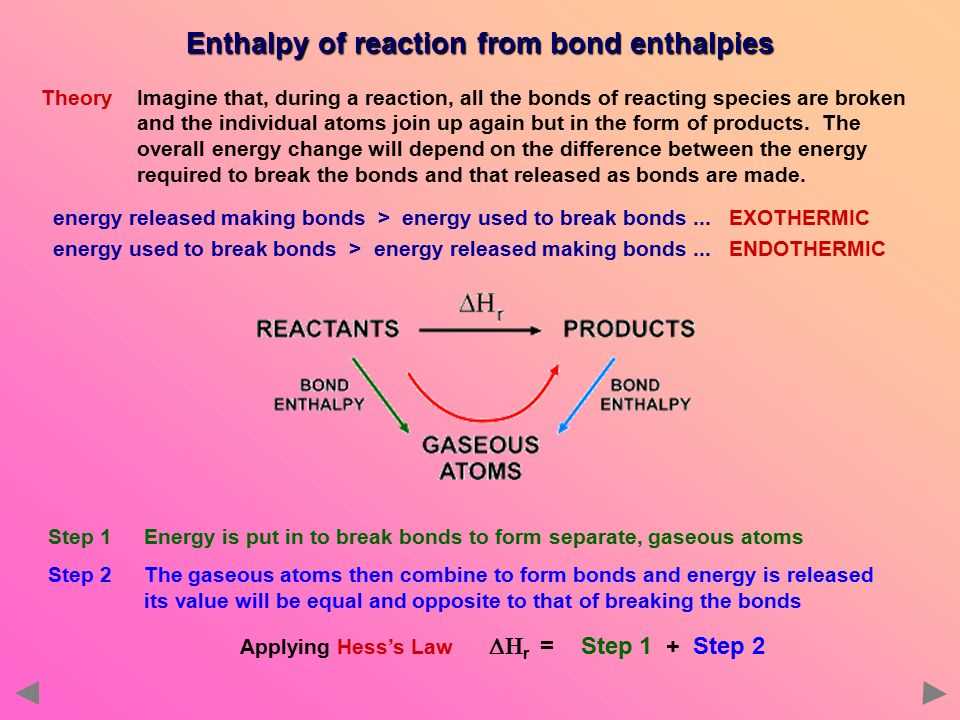
Another way to calculate enthalpy is through the use of Hess’s Law. Hess’s Law states that the enthalpy change of a reaction is independent of the pathway taken. By using a series of reactions with known enthalpy values, the enthalpy of a desired reaction can be calculated. This method is frequently used in chemistry to determine the enthalpy of formation or combustion of compounds.
In summary, enthalpy can be calculated using equations based on the internal energy, pressure, and volume of a system. Additionally, Hess’s Law can be used to determine the enthalpy change of a reaction by considering a series of reactions with known enthalpies. These calculations are fundamental in thermodynamics and provide valuable insight into the heat content and energy transfer of a system.
Understanding the standard enthalpy of formation
In chemistry, the standard enthalpy of formation (ΔH°f) is a measure of the heat energy released or absorbed when one mole of a compound is formed from its constituent elements in their standard states at a given temperature and pressure. It is an important concept in thermodynamics and is used to calculate the heat of reactions and the stability of compounds.
The standard states for elements are defined as their most stable form at a given temperature and pressure. For example, at room temperature and pressure, oxygen gas (O2) is in its standard state. The standard enthalpy of formation for elements in their standard states is zero.
The standard enthalpy of formation can be represented as a balanced chemical equation. The coefficients in the equation represent the number of moles of each compound involved in the reaction. The reactants are placed on the left side of the equation, and the products are on the right side.
By knowing the standard enthalpy of formation values for the reactants and products, we can calculate the change in enthalpy (ΔH) for a chemical reaction using the equation: ΔH = ΣnΔH°f(products) – ΣnΔH°f(reactants), where n represents the stoichiometric coefficients.
The standard enthalpy of formation is a useful tool in determining the energetics and feasibility of chemical reactions. It allows chemists to predict the heat released or absorbed during a reaction and assess the stability of compounds. By understanding the standard enthalpy of formation, scientists can make informed decisions about the energy requirements and potential applications of different chemical processes.
How enthalpy changes during chemical reactions
The concept of enthalpy is a fundamental concept in thermodynamics that helps us understand and quantify the energy changes that occur during chemical reactions. Enthalpy (H) is a state function that represents the heat content of a system. It includes both the internal energy (U) and the product of pressure (P) and volume (V) of the system.
During a chemical reaction, the enthalpy changes as a result of the breaking and forming of chemical bonds. When chemical bonds are broken, energy is required and the reaction is endothermic. The enthalpy change is positive in this case. Conversely, when new chemical bonds are formed, energy is released and the reaction is exothermic. The enthalpy change is negative in this case.
The enthalpy change can be calculated using Hess’s law or by using tabulated values called standard enthalpy of formation. Hess’s law states that the enthalpy change of a reaction is independent of the route taken, as long as there is no change in the initial and final states. This allows us to manipulate and combine equations to calculate the enthalpy change of a reaction.
The standard enthalpy of formation is the enthalpy change when one mole of a compound is formed from its elements in their standard states. It provides a convenient way to measure and compare the enthalpy changes of different reactions. The standard enthalpy of formation values are tabulated for various compounds.
In summary, enthalpy changes during chemical reactions depend on the breaking and forming of chemical bonds. The enthalpy change is positive when bonds are broken (endothermic) and negative when new bonds are formed (exothermic). Hess’s law and standard enthalpy of formation values are powerful tools for calculating and comparing enthalpy changes. Understanding enthalpy changes is crucial for predicting and controlling the energy flow in chemical reactions.
Applying the first law of thermodynamics to calculate enthalpy changes
The first law of thermodynamics states that energy can neither be created nor destroyed, only transferred or converted from one form to another. This law is fundamental in the field of thermodynamics and is particularly useful when calculating enthalpy changes in a system.
Enthalpy is a measure of the total heat energy in a system, and it includes the internal energy of the system as well as the work done on or by the system. Enthalpy changes occur during chemical reactions or phase transitions, and they can be calculated using the first law of thermodynamics.
When applying the first law of thermodynamics to calculate enthalpy changes, the equation used is ΔH = ΔU + PΔV, where ΔH is the change in enthalpy, ΔU is the change in internal energy, P is the pressure, and ΔV is the change in volume. This equation takes into account both the heat energy absorbed or released by the system and the work done on or by the system.
To calculate enthalpy changes, one must first determine the change in internal energy (ΔU) using the equation ΔU = q – PΔV, where q is the heat energy added or removed from the system and PΔV is the work done on or by the system.
Once the change in internal energy is determined, it can be added to PΔV to calculate the change in enthalpy (ΔH). If the value of ΔH is positive, it indicates that the reaction or phase transition is endothermic, meaning heat is absorbed by the system. Conversely, if the value of ΔH is negative, it indicates that the reaction or phase transition is exothermic, meaning heat is released by the system.
Practical examples of enthalpy calculations
Enthalpy calculations are an important tool in understanding and analyzing various physical and chemical processes. By quantifying the heat involved in these processes, enthalpy provides valuable insights into the energy transformations occurring.
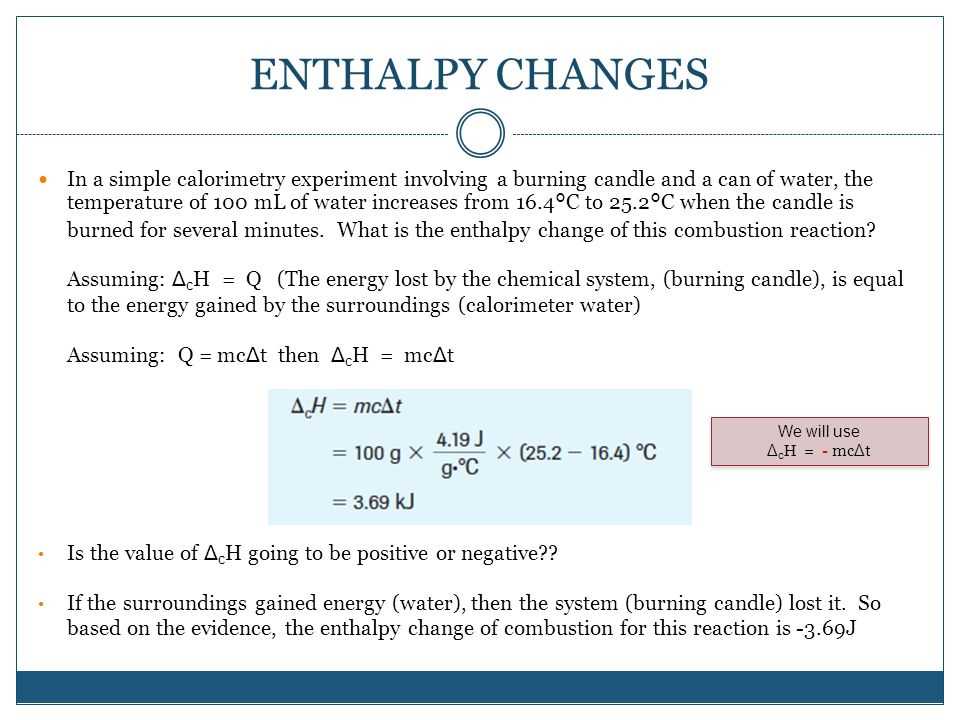
One practical example of enthalpy calculations is determining the heat required or released during a chemical reaction. The enthalpy change of a reaction, often denoted as ΔH, can be calculated by considering the enthalpy values of the reactants and products. For example, in the combustion of methane, the enthalpy change can be calculated by subtracting the sum of the enthalpies of the reactants (such as methane and oxygen) from the sum of the enthalpies of the products (such as carbon dioxide and water vapor).
Enthalpy calculations can also be utilized in determining the heat capacity of materials.
Another practical application of enthalpy calculations is in determining the heat capacity of materials. Heat capacity represents the amount of heat required to raise the temperature of a material by a certain amount. By measuring the temperature change and the heat transferred to or from the material, it is possible to calculate its heat capacity. This information is crucial in designing and optimizing heating and cooling systems, as well as understanding the thermal properties of substances.
In addition, enthalpy calculations are commonly used in thermodynamic processes, such as calculating the enthalpy of vaporization or fusion. These calculations involve determining the heat required to convert a substance from one phase to another. For example, to calculate the enthalpy of vaporization of water, one would measure the amount of heat required to convert a certain amount of liquid water into vapor at a constant temperature and pressure.
Enthalpy calculations are essential in various fields, including chemistry, physics, and engineering.
Enthalpy calculations have numerous practical applications and are employed in various fields. In chemistry, enthalpy calculations are essential in understanding and predicting the behavior of chemical reactions and designing efficient chemical processes. In physics, enthalpy calculations are used in thermodynamics to study the energy transformations in systems. In engineering, enthalpy calculations are crucial in designing and optimizing energy systems, such as power plants and HVAC systems.
Overall, enthalpy calculations provide a valuable tool for analyzing and understanding various physical and chemical processes. By quantifying the heat involved in these processes, enthalpy calculations enable more accurate predictions, efficient designs, and better control of energy transformations.
Common mistakes and pitfalls in enthalpy calculations
Enthalpy calculations can be complex and challenging, even for experienced chemists. There are several common mistakes and pitfalls to be aware of when performing enthalpy calculations.
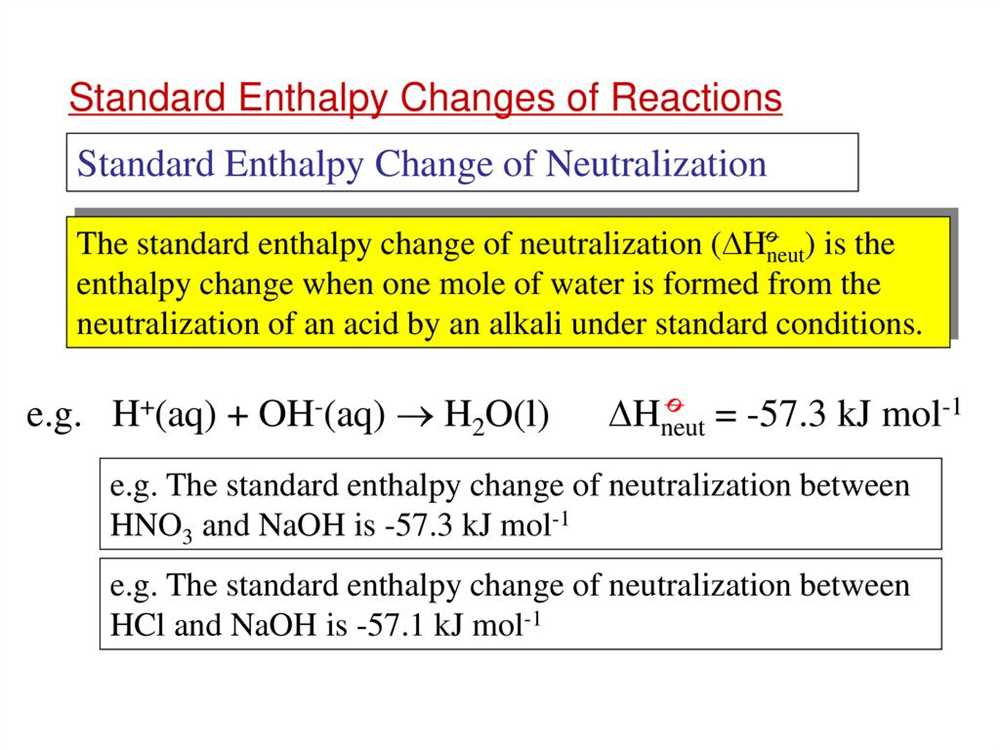
Incorrect sign of the enthalpy change: One common mistake is incorrectly determining the sign of the enthalpy change. The sign of the enthalpy change depends on whether the reaction is exothermic or endothermic. It is important to correctly identify the direction of heat flow in the reaction to determine the sign of the enthalpy change.
Misinterpretation of enthalpy values: Another pitfall is misinterpreting enthalpy values. Enthalpy values can be given in different units, such as joules or kilojoules, and it is important to use the correct units in calculations. Additionally, it is important to pay attention to whether enthalpy values are given per mole or per gram of a substance, as this will affect the calculations.
Failure to account for phase changes: One common mistake is failing to account for phase changes in enthalpy calculations. Phase changes, such as melting or vaporization, require the input or release of energy and therefore affect the overall enthalpy change of a reaction. It is important to consider the enthalpy changes associated with phase changes when performing calculations.
Ignoring the non-reacting substances: When calculating enthalpy changes, it is important to consider all substances involved in the reaction, including non-reacting substances. Non-reacting substances can contribute to the overall enthalpy change of a reaction and should not be ignored in the calculations.
Using incorrect stoichiometric coefficients: Finally, using incorrect stoichiometric coefficients can lead to incorrect enthalpy calculations. It is important to use the correct coefficients when balancing the chemical equation and performing enthalpy calculations.
Avoiding these common mistakes and pitfalls can help ensure accurate and reliable enthalpy calculations. It is important to double-check calculations and carefully consider all factors that can influence the enthalpy change of a reaction.