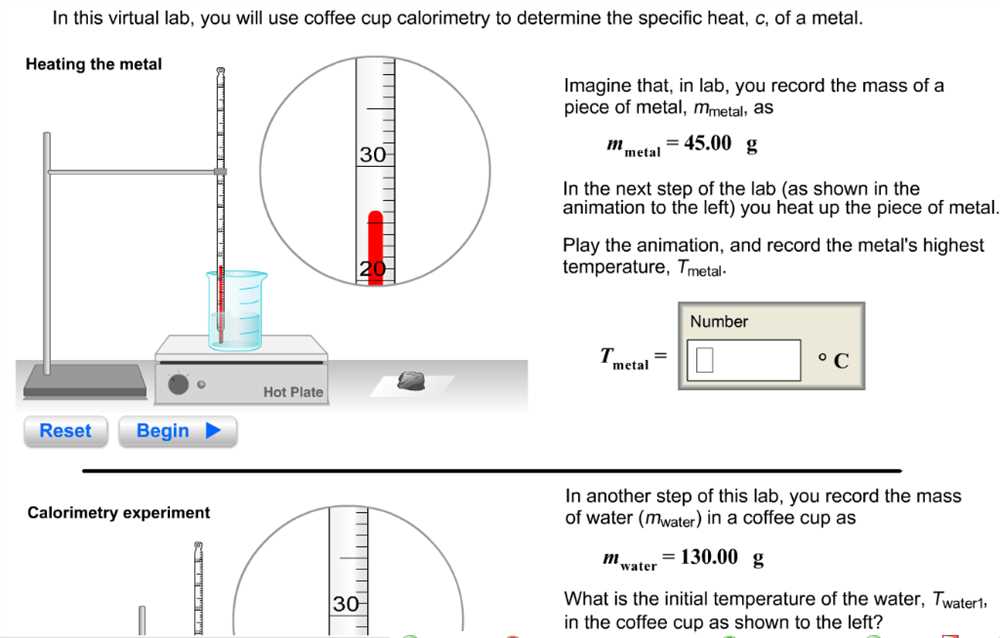
Calorimetry is a technique used to measure the amount of heat transferred during a chemical or physical process. In Experiment 25, students will be introduced to the principles of calorimetry and will perform a series of experiments to determine the heat absorbed or released by various substances.
The purpose of this pre-lab is to familiarize students with the concepts and calculations involved in calorimetry. By answering a set of pre-lab questions, students will demonstrate their understanding of the key principles and equations used in calorimetry experiments.
Some of the questions in this pre-lab include determining the specific heat capacity of a substance, calculating the heat absorbed or released during a reaction, and understanding the concept of enthalpy change. Students will also be asked to explain the importance of using a calorimeter in experiments and how to calculate the heat capacity of the calorimeter itself.
By completing this pre-lab, students will be prepared to conduct the Experiment 25 calorimetry lab with a solid understanding of the principles and calculations involved. This pre-lab serves as a valuable tool for ensuring that students are well-prepared and have the knowledge necessary to successfully perform the calorimetry experiments and analyze the results.
Experiment 25 Calorimetry Pre Lab Answers: Everything You Need to Know
In the field of chemistry, calorimetry is a technique used to measure the amount of heat generated or absorbed in a chemical reaction or physical process. It is an important tool for studying and understanding the energetics of various reactions. Experiment 25 focuses specifically on calorimetry and its applications.
In this pre-lab, you will find all the necessary answers and information to prepare for Experiment 25. It is essential to have a solid understanding of the concepts and procedures before conducting the actual experiment.
1. What is calorimetry?
Calorimetry is the science of measuring the heat of a chemical reaction or physical process. It involves the use of a calorimeter, which is an insulated container designed to minimize heat exchange with the surroundings. By measuring the change in temperature of a system, the heat transferred or exchanged can be determined.
2. How does calorimetry work?
Calorimetry works by measuring the temperature change of a system before and after a reaction. The heat transferred during the reaction causes a temperature change in the system, which can be measured using a thermometer. By knowing the specific heat capacity of the system and the temperature change, the amount of heat transferred can be calculated.
3. What is the purpose of Experiment 25?
The purpose of Experiment 25 is to demonstrate the principles of calorimetry and how it can be applied to measure the heat of reaction for different substances. By carrying out specific reactions and measuring the temperature change, students will be able to calculate the heat transfer of each reaction and compare the results.
4. What are the key components of a calorimeter?
A calorimeter typically consists of an insulated container, a lid with a thermometer, and a stirrer. The insulated container helps minimize heat exchange with the surroundings, ensuring accurate measurements. The lid with a thermometer allows for temperature monitoring, and the stirrer ensures uniform heat distribution.
5. What safety precautions should be taken during Experiment 25?
- Wear appropriate protective clothing, including gloves and goggles, to minimize the risk of exposure to chemicals.
- Handle chemicals with care and follow proper handling and disposal procedures.
- Avoid direct contact with hot surfaces or equipment.
- Use caution when handling glassware to prevent breakage or injury.
- Follow all instructions provided by the instructor or lab manual.
By reviewing these pre-lab answers, you will be well-equipped to carry out Experiment 25 and gain a deeper understanding of calorimetry and its applications in chemistry.
Overview of Experiment 25: Calorimetry
In Experiment 25: Calorimetry, we will be studying the transfer of heat energy between substances. Calorimetry is a technique used to measure the heat transfer during a chemical reaction or a physical change. The main purpose of this experiment is to determine the specific heat capacity of a metal sample.
The experiment will involve using a calorimeter, which is an insulated container, to measure the heat transfer between the metal sample and a known quantity of water. We will be measuring the initial and final temperatures of both the metal and the water, and using these values along with the mass and specific heat capacity of water to calculate the specific heat capacity of the metal.
To carry out the experiment, we will first calibrate the calorimeter by measuring the heat transfer between two samples of water at different temperatures. This will ensure that the calorimeter is properly insulated and that any heat loss is accounted for. Next, we will measure the initial temperature of the metal sample and the water and mix them together in the calorimeter. We will then measure the final temperature of the mixture and use this data to calculate the heat transfer and specific heat capacity of the metal.
This experiment provides valuable insight into the concept of heat transfer and the specific heat capacities of different substances. It also demonstrates the application of calorimetry as a technique for measuring heat transfer. By understanding the principles of calorimetry, we can gain a better understanding of thermodynamics and the behavior of substances at different temperatures.
Understanding Calorimetry: Definition, Process, and Importance
Calorimetry is a scientific measurement technique used to determine the heat transfer that occurs during a chemical reaction or physical process. It involves the use of a calorimeter, a device designed to accurately measure the change in temperature of a system as a result of a heat exchange.
The process of calorimetry involves measuring the heat absorbed or released by a substance under controlled conditions. This is done by placing the substance in a calorimeter and observing the change in temperature over a given period of time. The calorimeter acts as an isolated system, minimizing heat loss to the surroundings and ensuring accurate measurements. By monitoring the temperature change, scientists are able to calculate the amount of heat transferred.
Calorimetry is of great importance in various fields of science and engineering. In chemistry, it helps determine the energy content and calorific value of substances, which is essential for understanding their reactivity and potential applications. In physics, calorimetry is used to study thermal properties and investigate the behavior of materials at different temperatures. In biochemistry, it aids in studying metabolic reactions and understanding the energy balance of living organisms.
Calorimetry also plays a crucial role in the development of energy-efficient technologies and the design of sustainable processes. By accurately measuring the heat released or absorbed during a reaction or process, scientists and engineers can optimize energy consumption, improve efficiency, and reduce environmental impact.
Overall, calorimetry is a powerful tool in scientific research and engineering applications. Its ability to accurately measure heat transfer and energy content allows for a deeper understanding of chemical and physical processes and enables the development of more sustainable and efficient technologies.
The Purpose of Experiment 25: Calorimetry
Experiment 25: Calorimetry is conducted to measure the amount of heat absorbed or released during a chemical reaction. This experiment is essential in determining the enthalpy change, or heat of reaction, which provides valuable information about the energy changes occurring in a chemical system. By understanding these energy changes, scientists and researchers can better comprehend the nature of chemical reactions and the factors that influence their rates and efficiency.
Calorimetry is a technique used to measure the heat change associated with a chemical reaction or a physical process. In this experiment, a calorimeter is used to isolate the reaction and track the heat flow. The basic principle of calorimetry is that the heat given off or absorbed by the reaction is equal to the heat gained or lost by the surroundings.
One of the main purposes of Experiment 25 is to determine the heat capacity of the calorimeter. The heat capacity represents the amount of heat required to increase the temperature of the calorimeter by one degree Celsius. This value is crucial in accurately measuring the heat of reaction, as it allows for the calculation of the heat exchanged between the reaction and the surroundings.
Additionally, Experiment 25 aims to investigate the relationship between the heat of reaction and the molar quantities of reactants and products. By conducting multiple trials with different amounts of reactants, scientists can analyze how the heat of reaction varies with the changes in quantities. This information can be used to identify the stoichiometry of the reaction and determine the enthalpy change per mole of reactant.
In summary, Experiment 25: Calorimetry is performed to measure the heat of reaction and understand the energy changes that occur during a chemical reaction. Through this experiment, scientists can determine the heat capacity of the calorimeter, investigate the relationship between heat of reaction and mole quantities, and gain valuable insights into the thermodynamics of chemical systems.
Experimental Setup: Equipment and Materials Required

In order to carry out Experiment 25 on calorimetry, the following equipment and materials are required:
- Calorimeter: A calorimeter is used to measure the heat transfer during the experiment. It is typically made of an insulated container with a lid, which minimizes heat loss or gain to the surroundings.
- Stirring Device: A stirring device, such as a magnetic stirrer or a glass rod, is needed to ensure uniform mixing of the substances inside the calorimeter and promote heat transfer.
- Thermometer: A thermometer is used to measure the temperature changes during the experiment. It is typically placed inside the calorimeter to monitor the temperature of the substances being tested.
- Substances: The experiment may require specific substances to be tested for their heat transfer properties. These substances could be solids, liquids, or gases, depending on the specific objective of the experiment.
- Measuring Cylinder: A measuring cylinder is used to accurately measure the volumes of the substances being tested or the amounts of reagents to be mixed.
- Heat Source: Depending on the experiment, a heat source may be required to provide the initial energy input to the system. This could be a Bunsen burner, an electric heater, or any other suitable heat source.
- Data Recording Sheets: It is important to record the temperature readings and other relevant data during the experiment. Data recording sheets or a laboratory notebook should be available for this purpose.
It is essential to have all the above-mentioned equipment and materials in proper working condition before starting the experiment. Any discrepancies or malfunctions in the equipment could lead to inaccurate results or failed experiments. Additionally, the substances being tested should be handled with care and in accordance with the safety guidelines provided by the laboratory.
Step-by-Step Procedure for Conducting Experiment 25: Calorimetry
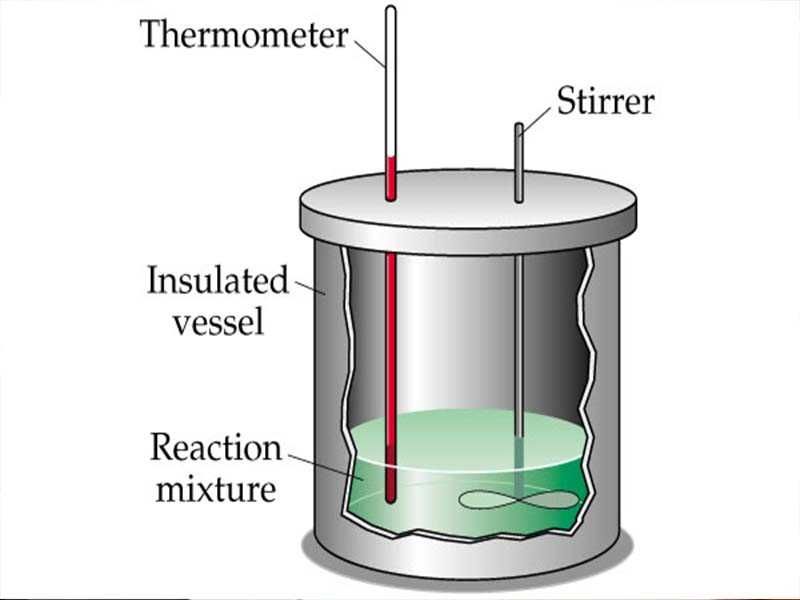
In this experiment, the goal is to determine the heat capacity of a calorimeter using the method of mixtures. This involves measuring the temperature change when two substances with different temperatures are mixed together.
Materials:
- Calorimeter
- Thermometer
- Hot water
- Cold water
- Stirrer
Procedure:
- Prepare the calorimeter by rinsing it with water to ensure it is clean and dry.
- Measure out a known mass of hot water and record its temperature using the thermometer. Repeat this step for the cold water.
- Empty the hot water into the calorimeter and record its initial temperature.
- Add the cold water to the calorimeter and stir gently with the stirrer to ensure thorough mixing.
- Record the highest temperature reached by the mixture.
- Calculate the heat gained by the cold water using the equation Q = mcΔT, where Q is the heat gained, m is the mass of the cold water, c is the specific heat capacity of water, and ΔT is the temperature change.
- Calculate the heat lost by the hot water using the same equation.
- Finally, calculate the heat capacity of the calorimeter using the equation C = (Q gained by cold water – Q lost by hot water) / ΔT, where C is the heat capacity and ΔT is the temperature change.
NOTE: It is important to handle the hot water with caution to avoid burns. Use proper protective equipment such as gloves and safety goggles. Repeat the experiment multiple times to ensure accurate results.
Data Collection and Analysis: How to Interpret the Results
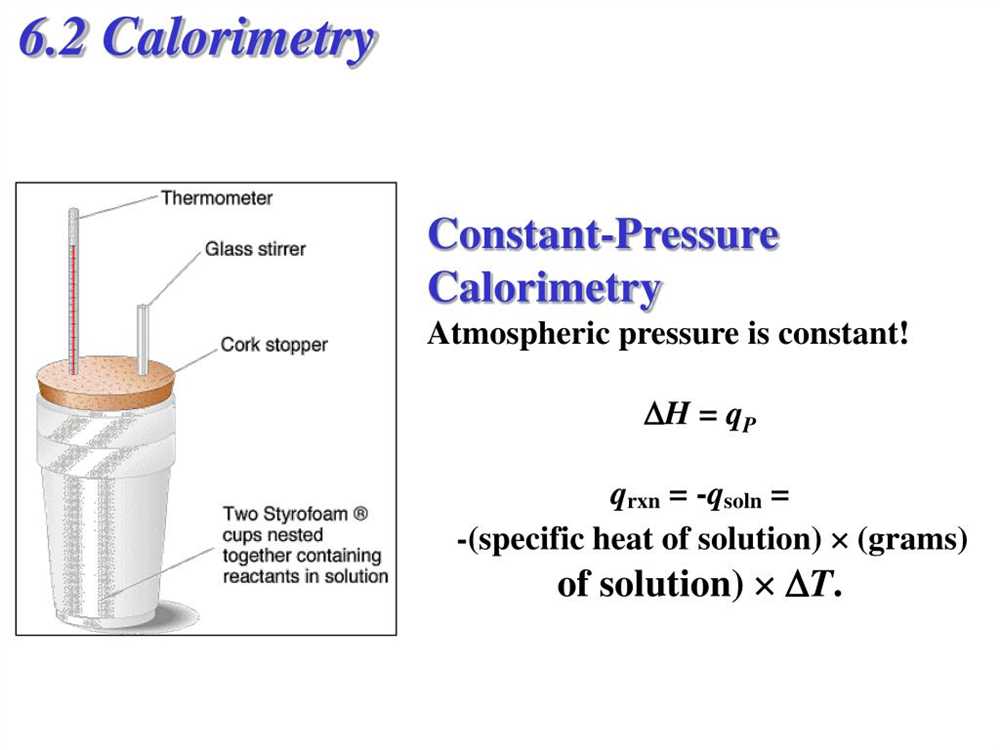
When conducting experiments, it is crucial to collect accurate and reliable data. The data collected during an experiment provides the basis for analysis and interpretation of the results. To effectively interpret the results, it is essential to understand the purpose and objectives of the experiment, as well as the specific variables being measured.
One important aspect of data collection is ensuring that the measurements are conducted using appropriate instruments and techniques. Accurate measurements are crucial for obtaining reliable data. Additionally, it is important to collect sufficient data points to ensure a statistically significant sample size.
Once the data has been collected, the next step is to analyze it. This involves organizing the data and identifying any patterns or trends. Graphs and charts can be useful tools for visualizing the data and identifying any relationships between variables.
Interpreting the results requires comparing the collected data to the expected or predicted outcomes. This involves analyzing any discrepancies or variations from the expected results and determining their possible causes. It may also involve performing statistical analyses to determine the significance of any observed differences.
Finally, it is important to draw meaningful conclusions from the data analysis. This involves making connections between the data and the original research question or hypothesis. It may also involve identifying areas for further investigation or suggesting potential applications or implications of the results.