Understanding the concept of Gibbs free energy is essential in studying thermodynamics and chemical reactions. To ascertain your understanding, it is crucial to practice solving Gibbs free energy problems using worksheets. In this article, we will provide comprehensive answers to a Gibbs free energy worksheet, explaining the step-by-step process and key concepts involved.
The worksheet prompts you to calculate the Gibbs free energy change (ΔG) for various chemical reactions. By determining ΔG, you can predict the spontaneity and direction of a reaction. Throughout the worksheet, you will encounter equations, values for enthalpy change (ΔH), entropy change (ΔS), and temperature (T). Utilizing the appropriate formula and appropriate units, you can solve for ΔG and unravel the thermodynamic information of the reaction.
Our answers to the worksheet will involve breaking down the calculations into smaller steps, ensuring a clear understanding of the concepts. We will emphasize the importance of correctly applying the formulas, manipulating units, and interpreting the results in the context of the reaction. By studying the answers provided, you will enhance your comprehension of Gibbs free energy and gain confidence in solving similar problems in the future.
Gibbs Free Energy Worksheet Answers
When answering a Gibbs Free Energy worksheet, it is important to understand the signs and values of ΔH and ΔS. If ΔG is negative, it means that the reaction is spontaneous and thermodynamically favorable. If ΔG is positive, it means that the reaction is non-spontaneous and thermodynamically unfavorable. When ΔG is zero, it means that the reaction is at equilibrium. Additionally, if ΔH is negative and ΔS is positive, the reaction is more likely to be spontaneous even at lower temperatures.
To calculate the value of ΔG, it is necessary to gather information about the enthalpy and entropy changes for the reaction. This may involve using standard enthalpy and entropy values for the reactants and products, or specific values given in the worksheet. It is also important to pay attention to the units of temperature used in the calculation, as it must be in Kelvin to match the units of entropy.
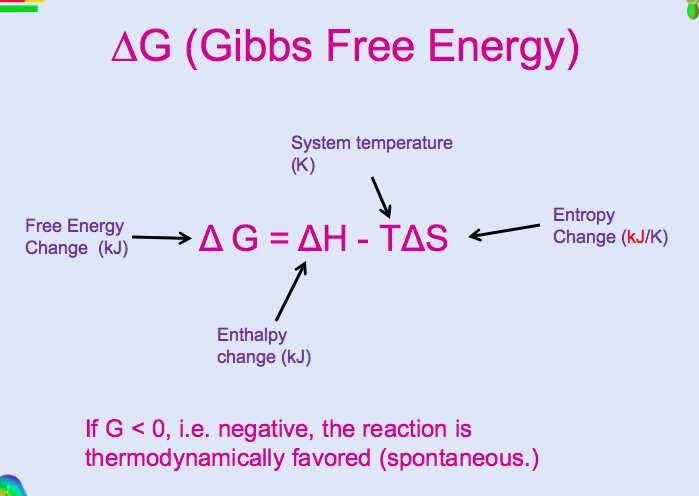
Overall, answering a Gibbs Free Energy worksheet requires a solid understanding of thermodynamics and the relationships between enthalpy, entropy, temperature, and spontaneity. It involves applying the equation ΔG = ΔH – TΔS, interpreting the signs and values of ΔH and ΔS, and performing calculations using the given information. It is an important skill for analyzing chemical reactions and determining their feasibility under different conditions.
What Is Gibbs Free Energy?
Gibbs free energy is a thermodynamic concept that measures the amount of energy available to do useful work in a chemical or physical process. It is named after the American scientist Josiah Willard Gibbs, who developed the concept in the late 19th century. Gibbs free energy is denoted by the symbol G and is the difference between the total energy of a system and its entropy multiplied by temperature:
G = H – TS
In this equation, H represents the enthalpy (total energy), T is the temperature in Kelvin, and S is the entropy (measure of disorder) of the system. The Gibbs free energy equation is commonly used in chemical thermodynamics to determine the spontaneity and feasibility of a reaction.
One of the key applications of Gibbs free energy is in determining whether a chemical reaction will occur spontaneously. If the Gibbs free energy change (ΔG) is negative, the reaction is thermodynamically favorable and will proceed spontaneously. On the other hand, if ΔG is positive, the reaction is not spontaneous and will require an input of energy to occur.
Gibbs free energy is also used to determine the equilibrium condition of a system. At equilibrium, the Gibbs free energy change is zero, indicating that the system is in a state of minimum energy and maximum stability. By calculating the Gibbs free energy change at different conditions, scientists can determine the optimal conditions for a reaction to reach equilibrium.
How to Calculate Gibbs Free Energy?
Gibbs free energy is an important thermodynamic property that helps determine whether a chemical reaction is spontaneous or not. It shows the maximum amount of useful work that can be extracted from a system at constant temperature and pressure. To calculate Gibbs free energy, several steps need to be followed:
- Identify the reaction: Write down the balanced chemical equation for the reaction you want to calculate the Gibbs free energy for.
- Determine the standard Gibbs free energy (ΔG°): This value can be found in standard thermodynamic tables for various compounds. Calculate the ΔG° for each compound involved in the reaction by subtracting the standard Gibbs free energy of the reactants from the standard Gibbs free energy of the products.
- Calculate the reaction quotient (Q): This is the ratio of the concentrations of the products to the concentrations of the reactants at any given point in the reaction. It can be calculated by dividing the product concentrations by the reactant concentrations.
- Calculate the actual Gibbs free energy (ΔG): Use the equation ΔG = ΔG° + RT ln(Q), where R is the gas constant (8.314 J/mol·K) and T is the temperature in Kelvin.
- Interpret the result: If the calculated ΔG is negative, the reaction is spontaneous and can occur without the need for external energy input. If ΔG is positive, the reaction is non-spontaneous and requires energy input to proceed.
Calculating Gibbs free energy is an essential part of understanding the feasibility of chemical reactions. It allows scientists to predict whether a reaction will occur spontaneously or not and provides insights into the thermodynamic stability of compounds. By following the steps outlined above, one can accurately calculate the Gibbs free energy and make informed predictions about the behavior of chemical systems.
Understanding Gibbs Free Energy Change
The value of ΔG can be used to determine whether a chemical reaction is spontaneous or non-spontaneous. If ΔG is negative, it means that the reaction is spontaneous and will occur without the need for an external driving force. On the other hand, if ΔG is positive, the reaction is non-spontaneous and will not occur without an external driving force. If ΔG is zero, it means that the system is at equilibrium.
In order to calculate ΔG, it is necessary to know the values of ΔH and ΔS for the reaction. ΔH represents the change in enthalpy, which is the heat absorbed or released during a chemical reaction. ΔS represents the change in entropy, which is a measure of the disorder or randomness in a system. A positive value of ΔS indicates an increase in disorder, while a negative value indicates a decrease in disorder.
By understanding the concept of Gibbs free energy change, scientists and engineers can predict whether a reaction will occur spontaneously and determine the conditions under which a reaction is most favorable. This knowledge is essential for designing and optimizing chemical processes and reactions.
Relationship Between Gibbs Free Energy and Equilibrium

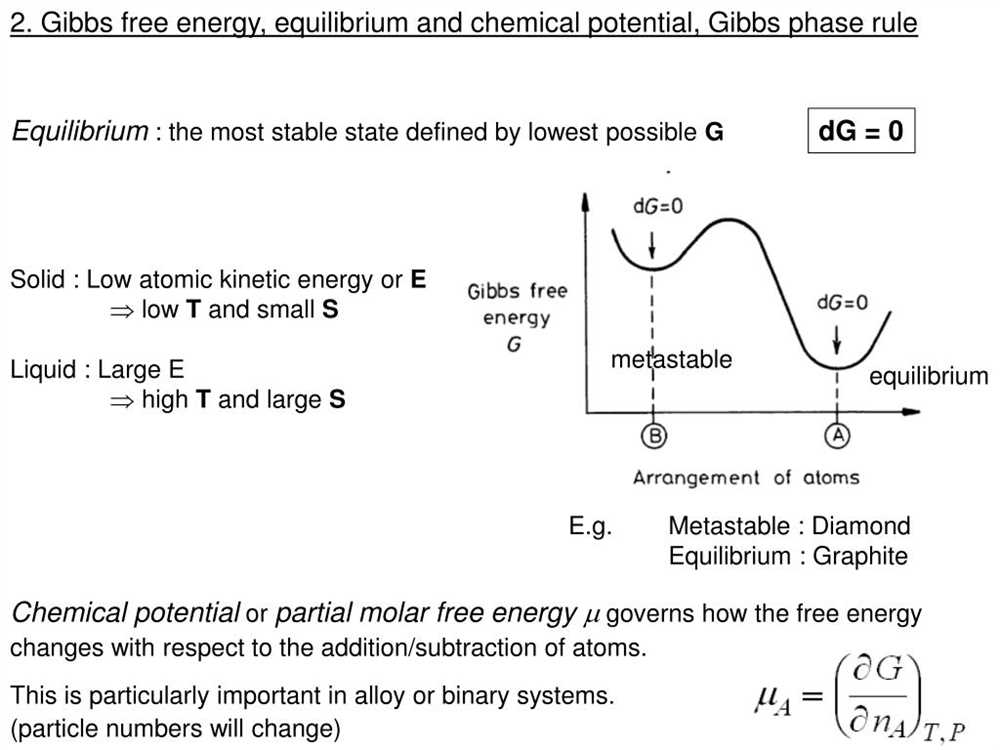
Gibbs free energy is a thermodynamic property that characterizes the spontaneity and directionality of a chemical reaction. It quantifies the maximum useful work that can be extracted from a system at constant temperature and pressure. Gibbs free energy is also closely related to the concept of equilibrium, which describes the state where the forward and reverse reactions of a chemical process occur at equal rates.
At equilibrium, the Gibbs free energy of a system is at its minimum value. This means that there is no net change in the system and the reaction has reached a state of balance. The Gibbs free energy change (∆G) for a reaction can be used to determine whether the reaction is spontaneous or non-spontaneous. If ∆G is negative, the reaction is spontaneous and can proceed in the forward direction. On the other hand, if ∆G is positive, the reaction is non-spontaneous and will not occur without the input of energy.
The relationship between Gibbs free energy and equilibrium can be further understood by considering the equation ∆G = ∆G° + RTln(Q), where ∆G° is the standard Gibbs free energy change, R is the gas constant, T is the temperature, and Q is the reaction quotient. At equilibrium, the reaction quotient is equal to the equilibrium constant (K), which allows the equation to be simplified to ∆G = ∆G° + RTln(K).
This equation demonstrates that the value of ∆G depends on the difference between the standard Gibbs free energy change (∆G°) and the natural logarithm of the equilibrium constant (RTln(K)). A negative ∆G indicates that the reaction is spontaneous and will proceed towards equilibrium. Conversely, a positive ∆G suggests that the reaction is non-spontaneous and will not reach equilibrium under the given conditions.
In summary, Gibbs free energy provides valuable insights into the spontaneity and directionality of chemical reactions. It is closely tied to the concept of equilibrium, with the value of ∆G indicating the tendency for a reaction to proceed towards or away from equilibrium. Understanding the relationship between Gibbs free energy and equilibrium is fundamental in predicting the behavior of chemical systems and designing efficient processes in industries such as chemistry, biology, and engineering.
Examples of Gibbs Free Energy Calculations
The Gibbs free energy, or Gibbs function, is a thermodynamic potential that measures the maximum reversible work that can be performed by a system at constant temperature and pressure. It is commonly used to determine the feasibility of a chemical reaction or the stability of a system.
One example of Gibbs free energy calculations is determining the spontaneity of a chemical reaction. By comparing the Gibbs free energy change (∆G) of the reaction to zero, we can determine if the reaction is favorable or not. If ∆G is negative, the reaction is exergonic and spontaneous. If ∆G is positive, the reaction is endergonic and non-spontaneous. For example, consider the reaction A + B -> C + D. If ∆G is calculated to be -10 kJ/mol, we can conclude that the reaction is spontaneous.
Another example is calculating equilibrium constants using Gibbs free energy. The equilibrium constant (K) is a measure of the ratio of the concentrations of products to reactants at equilibrium. By using the equation ∆G = -RTlnK, where R is the gas constant and T is the temperature, we can calculate the equilibrium constant from the Gibbs free energy change. For example, if we have a reaction with ∆G = -20 kJ/mol and T = 298 K, we can calculate the equilibrium constant by rearranging the equation to K = e^(-∆G/RT).
Gibbs free energy calculations are also used in predicting the stability of a system. For example, in electrochemical cells, the Gibbs free energy change can be used to determine the electrode potential and the direction of electron flow. By comparing the Gibbs free energies of the electrodes, we can predict which electrode will be reduced and which will be oxidized. This information is crucial in designing and optimizing battery systems.
In conclusion, Gibbs free energy calculations are a valuable tool in understanding the thermodynamics of chemical reactions and the stability of systems. They can be used to determine the spontaneity of reactions, calculate equilibrium constants, and predict the direction of electron flow in electrochemical cells. These calculations provide valuable insights into the feasibility and efficiency of chemical processes.
Gibbs Free Energy and Spontaneity
Gibbs free energy is a concept in thermodynamics that measures the spontaneity or feasibility of a chemical reaction or physical process. It is denoted by the symbol ΔG and is defined as the change in Gibbs free energy between the initial and final states of a system. Gibbs free energy takes into account both the enthalpy (heat) and entropy (disorder) changes of a system, allowing us to predict whether a reaction will proceed spontaneously or requires an input of energy.
A negative ΔG value indicates that a reaction is spontaneous and will proceed in the forward direction. This means that the reaction releases energy and the system becomes more stable. On the other hand, a positive ΔG value indicates that a reaction is non-spontaneous and will not proceed in the forward direction without an input of energy. In this case, the reaction is endothermic and the system becomes less stable.
The relationship between ΔG and spontaneity can be summarized as follows:
- If ΔG < 0, the reaction is spontaneous.
- If ΔG = 0, the reaction is at equilibrium.
- If ΔG > 0, the reaction is non-spontaneous.
The value of ΔG can also be used to determine the direction in which a reaction will proceed. If ΔG is negative, the reaction will proceed in the forward direction. If ΔG is positive, the reaction will proceed in the reverse direction. The magnitude of ΔG also provides information about the spontaneity of the reaction; the greater the magnitude of ΔG, the more spontaneous the reaction.
In summary, Gibbs free energy is a useful tool in determining the spontaneity and direction of chemical reactions. By calculating the ΔG value, we can predict whether a reaction will occur spontaneously and understand the thermodynamic feasibility of a process.
Gibbs Free Energy and Chemical Reactions
Gibbs free energy (G) is a thermodynamic quantity that determines the spontaneity and the extent of a chemical reaction. It is used to predict whether a reaction will occur spontaneously under certain conditions. The Gibbs free energy of a system is defined as the maximum amount of non-expansion work that can be extracted from the system at constant temperature and pressure.
The Gibbs free energy change (ΔG) of a reaction is a measure of the difference in Gibbs free energy between the products and the reactants. A negative ΔG indicates that the reaction is spontaneous and will proceed in the forward direction. A positive ΔG indicates that the reaction is non-spontaneous and will not proceed in the forward direction without the input of external energy.
One way to calculate the Gibbs free energy change of a reaction is by using the equation ΔG = ΔH – TΔS, where ΔH is the enthalpy change of the reaction, ΔS is the entropy change of the reaction, and T is the temperature in Kelvin. If ΔG is negative, the reaction is spontaneous at that temperature. If ΔG is positive, the reaction is non-spontaneous at that temperature.
Example:
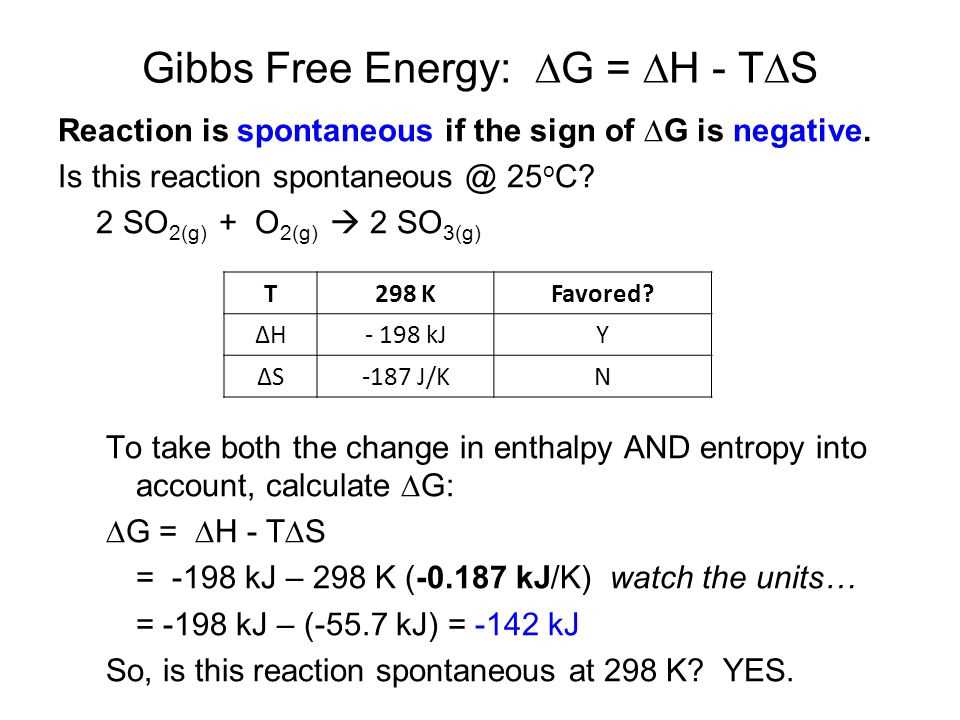
Let’s consider the reaction A + B → C. The enthalpy change ΔH is -100 kJ/mol and the entropy change ΔS is 50 J/(mol·K). The temperature is 298 K. Using the equation ΔG = ΔH – TΔS, we can calculate the Gibbs free energy change:
- Convert ΔH to J/mol: -100 kJ/mol = -100,000 J/mol.
- Plug the values into the equation: ΔG = -100,000 J/mol – (298 K)(50 J/(mol·K)).
- Calculate the ΔG: ΔG = -100,000 J/mol – 14,900 J/mol.
- The ΔG is -115,100 J/mol.
Since ΔG is negative, the reaction A + B → C is spontaneous at 298 K.
Gibbs free energy is a useful concept in chemistry to understand the spontaneity and direction of chemical reactions. By calculating the ΔG of a reaction, we can determine whether it will occur spontaneously and in which direction. This information is crucial in determining the feasibility of chemical processes and designing efficient reactions.