When it comes to chemistry, one of the fundamental skills that every student must acquire is the ability to balance chemical equations. This process involves ensuring that the number of atoms of each element on both sides of the equation is equal, thus following the law of conservation of mass.
The Gizmo Balancing Chemical Equations Answer Key is a valuable tool that assists students in mastering this essential skill. This interactive online resource provides step-by-step instructions and practice problems to help learners understand the process of balancing chemical equations.
With the Gizmo Balancing Chemical Equations Answer Key, students can explore different equations and experiment with coefficients to achieve balance. They can also learn about the various types of reactions, such as synthesis, decomposition, and combustion, and see how the number of atoms changes during these reactions.
By using the Gizmo Balancing Chemical Equations Answer Key, students can develop a solid foundation in stoichiometry and chemical reactions. This knowledge is crucial for success in advanced chemistry courses and for understanding the principles behind many everyday processes, such as combustion, digestion, and respiration.
Gizmo Balancing Chemical Equations Answer Key
In chemistry, balancing chemical equations is a fundamental skill that allows us to understand the reactions taking place. The Gizmo Balancing Chemical Equations Answer Key is a useful tool for students to check their work and ensure they have balanced the equations correctly. By using this answer key, students can compare their solutions with the correct answers provided.
The answer key provides step-by-step instructions on how to balance each equation in the Gizmo Balancing Chemical Equations activity. It includes the coefficients needed for each reactant and product to balance the equation. Students can use this key to verify their own answers and identify any mistakes they may have made.
The Gizmo Balancing Chemical Equations Answer Key is an invaluable resource for students learning chemistry. It helps them practice and reinforce their understanding of balancing chemical equations, which is crucial for success in chemistry. By using this answer key, students can gain confidence in their abilities and improve their problem-solving skills.
What is a chemical equation?
A chemical equation is a symbolic representation of a chemical reaction. It shows the reactants, which are the substances that undergo a change, and the products, which are the resulting substances. The equation indicates the number of molecules or atoms involved in the reaction using coefficients and formulas.
In a chemical equation, the reactants are written on the left side and the products are written on the right side, separated by an arrow. The arrow points from the reactants to the products, indicating the direction of the reaction. The coefficients in front of the formulas indicate the relative amounts of each substance, while the formulas themselves represent the composition of the substances.
A chemical equation follows the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction. This means that the number and types of atoms must be the same on both sides of the equation. To achieve this balance, coefficients can be adjusted to ensure that the number of atoms of each element is equal on both sides.
Example of a chemical equation:
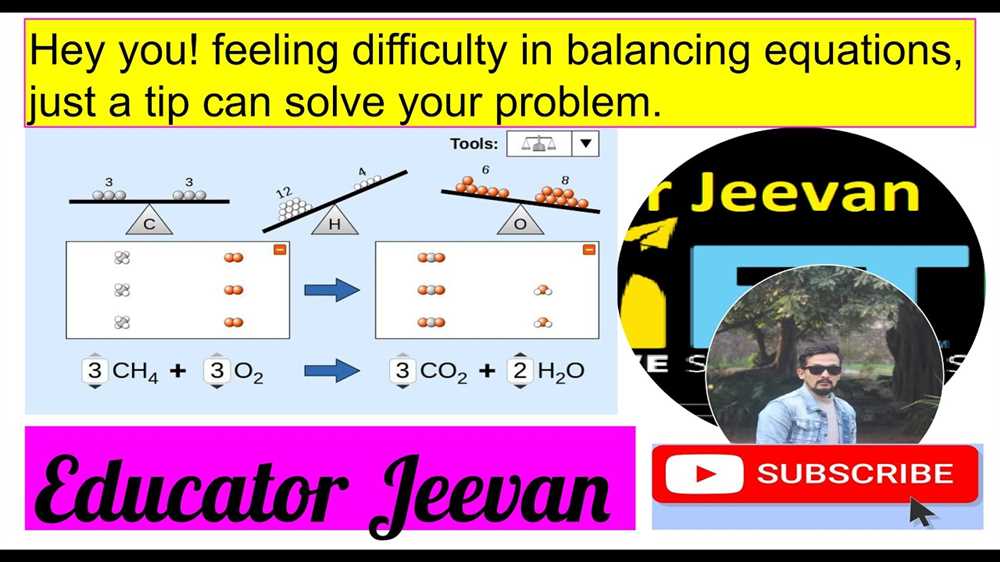
- Reactants: 2H2 + O2
- Product: 2H2O
This equation represents the reaction between hydrogen gas (H2) and oxygen gas (O2) to produce water (H2O). The balanced equation shows that two molecules of hydrogen react with one molecule of oxygen to form two molecules of water.
Chemical equations are essential for understanding and predicting chemical reactions. They provide a concise and efficient way to represent the changes that occur at the atomic or molecular level. By balancing the equation, scientists can determine the stoichiometry of the reaction, which helps in determining the amounts of reactants needed or products formed.
Why is balancing chemical equations important?

Balancing chemical equations is a fundamental skill in chemistry that is important for several reasons. Firstly, it ensures that there is a conservation of mass in a chemical reaction. In other words, the total number of atoms of each element on both sides of the equation remains the same. Balancing the equation allows us to accurately represent the reactants and products involved in the reaction.
Additionally, balancing chemical equations allows chemists to determine the stoichiometry of a reaction. Stoichiometry refers to the quantitative relationship between the amounts of substances involved in a chemical reaction. By balancing the equation, we can determine the exact ratios of reactants and products, which is crucial for determining the yield of a reaction or predicting how much of a particular reactant is needed to produce a desired amount of product.
Furthermore, balanced chemical equations provide a basis for calculating the theoretical yield and percent yield of a reaction. The theoretical yield is the maximum amount of product that can be obtained from a given amount of reactant, while the percent yield is a measure of the efficiency of the reaction. Balancing the equation allows us to accurately calculate these values and assess the success of a reaction.
In summary, balancing chemical equations is important because it ensures the conservation of mass, allows for the determination of stoichiometry, and provides a basis for calculations such as theoretical yield and percent yield. It is an essential skill for understanding and predicting the outcomes of chemical reactions.
How to Balance Chemical Equations
In chemistry, chemical equations are used to represent reactions between different substances. These equations show the starting substances, called reactants, and the resulting substances, called products. However, chemical equations need to be balanced in order to accurately represent the reaction. Balancing an equation involves adjusting the coefficients in front of the reactants and products so that the number of atoms of each element on both sides of the equation is equal.
To balance a chemical equation, start by identifying the elements that appear on both sides of the equation. Then, choose one element at a time and adjust the coefficients in front of the reactant or product that contains that element. The goal is to have an equal number of atoms for each element on both sides of the equation.
When balancing equations, it is important to remember that you can only adjust the coefficients, not the subscripts. Changing the subscripts would represent a different molecule and a different reaction entirely. Instead, you must find the right combination of coefficients that will balance the equation.
One common strategy for balancing equations is to start with the most complex molecules or the molecules with the highest number of atoms. By adjusting their coefficients first, it can be easier to balance the rest of the equation. Additionally, it can be helpful to keep a tally of the number of atoms for each element on both sides of the equation to ensure that they remain equal throughout the balancing process.
Overall, balancing chemical equations requires patience and practice. It is an important skill in chemistry as it allows scientists to accurately represent reactions and understand the stoichiometry of chemical reactions. By following the steps mentioned above, anyone can learn how to balance chemical equations effectively.
Using the Gizmo Balancing Chemical Equations tool
The Gizmo Balancing Chemical Equations tool is a valuable resource for students learning about chemical reactions and how to balance them. This interactive tool allows users to practice balancing chemical equations by dragging and dropping coefficients in front of the reactants and products. It provides a visual representation of the chemical equation and allows for hands-on experimentation.
One of the key features of the Gizmo Balancing Chemical Equations tool is the ability to see the equation in both its balanced and unbalanced forms. This allows students to compare and contrast the two and understand the importance of balancing chemical equations. By manipulating the coefficients, users can observe how changes affect the equation and the quantities of each element involved.
The Gizmo Balancing Chemical Equations tool also offers hints and feedback to guide students in the right direction. If a user attempts to balance the equation incorrectly, the tool will provide suggestions for corrections. This helps students learn from their mistakes and improve their skills in balancing chemical equations.
Furthermore, the Gizmo Balancing Chemical Equations tool includes a variety of chemical reactions for students to practice on. These reactions range from simple to complex, allowing users to gradually strengthen their understanding of balancing chemical equations. The tool also provides a step-by-step solution for each equation, enabling students to see the correct method for balancing the equation.
In conclusion, the Gizmo Balancing Chemical Equations tool is an effective educational resource for students studying chemical reactions. Its interactive nature, visual representation, hints, and diverse range of chemical reactions make it an engaging and valuable tool for learning how to balance chemical equations accurately.
Step-by-step guide to balancing equations with Gizmo

When it comes to balancing chemical equations, the Gizmo tool can be a valuable resource. With its interactive interface and step-by-step instructions, it makes the process of balancing equations much simpler and more engaging.
The first step in balancing equations with the Gizmo is to input the chemical equation you want to balance. This can be done by typing the chemical formulas and coefficients into the appropriate fields. Once the equation is entered, the Gizmo will display the unbalanced equation and provide a list of available elements and compounds.
Next, you can begin the process of balancing the equation. The Gizmo will guide you through this step by step, starting with the lowest coefficient in the equation. You can adjust the coefficients by using the arrow buttons or by typing in the desired value. As you make changes, the Gizmo will automatically update the equation and display the result.
As you continue to balance the equation, you may encounter situations where multiple coefficients need to be adjusted simultaneously. In these cases, the Gizmo will highlight the affected coefficients and provide suggestions for balancing the equation. You can choose to follow these suggestions or come up with your own solution.
Once all the coefficients are adjusted and the equation is balanced, the Gizmo will display the final balanced equation. You can then review the changes you made and see how they affected the equation. The Gizmo also provides a step-by-step explanation of the balancing process, allowing you to understand the logic behind each adjustment.
In conclusion, the Gizmo tool offers a user-friendly and interactive way to balance chemical equations. It simplifies the process and provides clear instructions, making it easier for students and professionals alike to master this fundamental skill in chemistry.
Common challenges in balancing chemical equations
When it comes to balancing chemical equations, there are several common challenges that students and even professionals face. Balancing equations is a fundamental skill in chemistry, as it ensures that the law of conservation of mass is upheld and all reactants and products are accounted for. However, it can be a complex process that requires attention to detail and a thorough understanding of the reaction.
1. Differentiating between coefficients and subscripts: One of the main challenges in balancing chemical equations is distinguishing between coefficients and subscripts. Coefficients are used to balance the number of atoms or molecules of each element on both sides of the equation, while subscripts represent the number of atoms within a molecule. It is crucial to recognize the difference between these two and correctly adjust the coefficients to achieve a balanced equation.
2. Dealing with complex reactions: Another challenge arises when balancing complex reactions that involve multiple reactants and products. These reactions may have numerous compounds and elements, making it more difficult to determine the appropriate coefficients. It requires careful analysis of each element’s presence and its overall contribution to the reaction to achieve a balanced equation.
3. Dealing with fractional coefficients: Sometimes, balancing chemical equations may result in the need for fractional coefficients. While it is ideal to have whole numbers as coefficients, there are cases where fractional coefficients are necessary to balance the equation correctly. This can be confusing for students who are accustomed to working with whole numbers and may require additional explanation and practice.
4. Complex stoichiometry: Stoichiometry is the calculation of the quantities of reactants and products in a chemical reaction. In some cases, balancing an equation requires considering stoichiometric ratios, which can introduce additional complexities. Calculating stoichiometric ratios accurately is crucial in balancing chemical equations and ensuring the conservation of mass.
5. Practice and perseverance: Balancing chemical equations is a skill that requires practice and perseverance. It may take time and multiple attempts to achieve a balanced equation, especially for more complex reactions. Regular practice, understanding the underlying principles, and seeking guidance when needed are essential for mastering this skill.
In conclusion, balancing chemical equations can be challenging due to various factors such as differentiating between coefficients and subscripts, handling complex reactions, dealing with fractional coefficients, considering complex stoichiometry, and the need for practice and perseverance. Developing a strong foundation in these areas and continuously honing the skill of balancing equations is crucial for success in chemistry.