
Understanding covalent bonds is an essential part of studying chemistry, and Gizmos provides students with an interactive platform to explore this concept. With a range of activities and simulations, students can enhance their learning experience and gain a deeper understanding of covalent bonding.
In the Gizmos Student Exploration Covalent Bonds module, students are introduced to the concept of covalent bonding, where atoms share electrons to form stable molecules. Through hands-on activities, students can manipulate atoms and observe how the number of shared electrons affects the strength and stability of the bond.
One of the key features of the Gizmos Covalent Bonds module is the interactive simulations that allow students to experiment with different atoms and observe the resulting bonds. This hands-on approach helps students visualize the molecular structure and understand how different elements combine to form compounds.
By using Gizmos Student Exploration Covalent Bonds, students can test their understanding of covalent bonding through various activities and assessments. They can explore the factors that affect bond strength, such as the type of atom and the number of shared electrons. Additionally, the module provides detailed explanations and answers to help students grasp the concepts and reinforce their learning.
Overall, Gizmos Student Exploration Covalent Bonds offers an engaging and interactive learning experience for students studying chemistry. By providing a platform to explore and manipulate atoms, students can develop a deeper understanding of covalent bonding and gain valuable insights into the molecular world.
Gizmos Student Exploration Covalent Bonds Answers
Covalent bonds are a type of chemical bond where two atoms share electrons. This type of bond is common in molecules made up of nonmetal atoms. The Gizmos Student Exploration Covalent Bonds activity allows students to explore the formation of covalent bonds by manipulating atoms and observing the resulting molecules. The activity provides a set of questions that students can use to guide their exploration and deepen their understanding of covalent bonding.
One of the questions in the activity asks students to determine the number of valence electrons in a given atom. Valence electrons are the electrons in the outermost energy level of an atom and are responsible for forming chemical bonds. To find the number of valence electrons, students can look at the group number of the element on the periodic table. For example, carbon is in group 14, so it has 4 valence electrons. Oxygen is in group 16, so it has 6 valence electrons.
Another question in the activity asks students to predict the formula of a molecule based on the atoms present and the number of electrons needed for a stable configuration. Students can use the octet rule as a guide to determine the number of electrons needed for a stable configuration. Most atoms tend to gain, lose, or share electrons in order to achieve a full outer shell with 8 electrons. Students can count the valence electrons of each atom and then determine how many more electrons are needed to reach 8. The formula of the molecule can then be determined based on the number of atoms present and the number of electrons needed for a stable configuration.
The Gizmos Student Exploration Covalent Bonds activity provides a hands-on and interactive way for students to explore the concept of covalent bonding. By manipulating atoms and observing the resulting molecules, students can deepen their understanding of this important chemical bond. The activity also encourages critical thinking and problem-solving skills by asking students to predict and analyze the properties of molecules based on their structures and electron configurations.
- Covalent bonds: a type of chemical bond where two atoms share electrons
- Valence electrons: the electrons in the outermost energy level of an atom, responsible for forming chemical bonds
- Octet rule: atoms tend to gain, lose, or share electrons in order to achieve a full outer shell with 8 electrons
- Formula of a molecule: determined based on the number of atoms present and the number of electrons needed for a stable configuration
What are Covalent Bonds?
A covalent bond is a type of chemical bond that forms when two atoms share electrons. Unlike ionic bonds, where electrons are transferred from one atom to another, covalent bonds involve the sharing of electrons between atoms. This sharing allows both atoms to fill their outermost electron shells and achieve a stable electron configuration.
In a covalent bond, the shared electrons are attracted to the nuclei of both atoms, forming a strong bond between them. The electrons are shared in such a way that the resulting molecule has a stable and balanced charge. Covalent bonds can occur between atoms of the same element, such as in diatomic molecules like oxygen (O2) or nitrogen (N2), or between different elements, as in molecules like methane (CH4) or water (H2O).
Covalent bonds can be classified as either polar or nonpolar. In a polar covalent bond, the shared electrons are not equally distributed between the two atoms, resulting in a partial positive charge on one atom and a partial negative charge on the other. This occurs when one atom has a higher electronegativity, or electron-attracting ability, than the other. In a nonpolar covalent bond, the shared electrons are equally distributed, resulting in no charge separation.
Covalent bonds play a crucial role in the formation of molecules and the interactions between atoms. They are responsible for the stability of compounds and the diversity of organic molecules found in nature. Understanding covalent bonds and their properties is essential for studying the structure and behavior of molecules and for many applications in chemistry and biology.
Properties of Covalent Bonds
Covalent bonds are a type of chemical bond formed when two atoms share electrons. They are commonly found in molecules and compounds that contain nonmetal elements, such as carbon, hydrogen, nitrogen, and oxygen. Covalent bonds have several distinct properties that make them unique and important in many aspects of chemistry.
1. Strong and Stable: Covalent bonds are strong and stable, holding atoms together to form molecules with a relatively high degree of strength. This is due to the sharing of electrons between atoms, creating a bond that requires a significant amount of energy to break.
2. Localization of electrons: In covalent bonds, electrons are shared between atoms, creating localized electron pairs that significantly impact the properties of the molecules. The arrangement of these electron pairs determines the overall shape and polarity of the molecule, which in turn affects its chemical and physical properties.
3. Variable bond strength: Covalent bonds can have different strengths depending on the types of atoms involved and the number of shared electron pairs. Some covalent bonds are stronger than others, and this bond strength affects the stability and reactivity of the molecule.
4. Non-conductivity of electricity: Covalent bonds do not conduct electricity in their pure form. Since electrons are shared between atoms in a localized manner, there are no free-moving charged particles to carry an electric current.
5. Low boiling and melting points: Covalent compounds generally have lower boiling and melting points compared to ionic compounds. This is because the intermolecular forces between covalent molecules are weaker than the electrostatic forces between ions in an ionic compound.
6. Solubility in nonpolar solvents: Many covalent compounds are soluble in nonpolar solvents, such as hydrocarbons, due to their similar molecular structures. This is because nonpolar solvents can interact with and dissolve the nonpolar covalent molecules.
Overall, covalent bonds are essential for the formation and stability of a wide range of molecules and compounds, playing a crucial role in various chemical and biological processes. Understanding the properties of covalent bonds allows scientists to predict and manipulate the behavior of these substances, leading to advancements in fields such as medicine, materials science, and environmental chemistry.
Types of Covalent Bonds
In chemistry, covalent bonds are formed when two atoms share electrons in order to achieve a stable electron configuration. There are three main types of covalent bonds: single, double, and triple bonds. Each type of bond is characterized by the number of electron pairs shared between the atoms.
1. Single Bonds:
A single bond is formed when two atoms share one pair of electrons. This is the most common type of covalent bond. In a single bond, the two atoms are held together by the electrostatic attraction between the shared electrons and the positively charged nuclei of the atoms. Single bonds are relatively weak and can be easily broken.
2. Double Bonds:
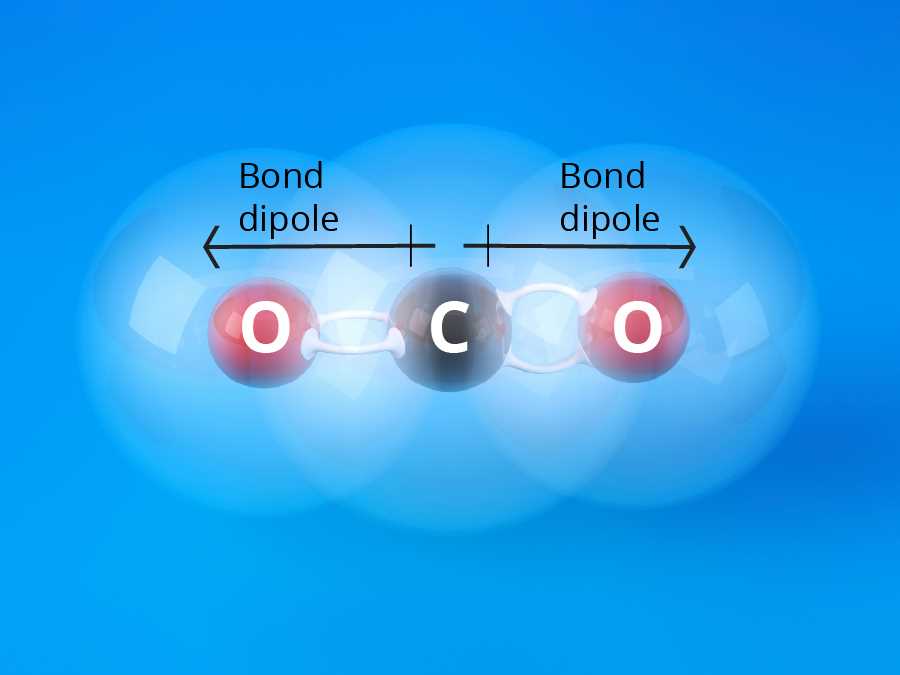
A double bond is formed when two atoms share two pairs of electrons. Double bonds are stronger than single bonds due to the increased number of shared electrons. The atoms in a double bond are held together by a stronger electrostatic attraction. Double bonds are often found in molecules with carbon, such as ethene (C2H4).
3. Triple Bonds:
A triple bond is formed when two atoms share three pairs of electrons. Triple bonds are the strongest type of covalent bond. The atoms in a triple bond are held together by a very strong electrostatic attraction. Triple bonds are commonly found in molecules with nitrogen, such as nitrogen gas (N2).
In summary, the types of covalent bonds, single, double, and triple bonds, differ in the number of electron pairs shared between the atoms. Single bonds involve one pair of shared electrons, double bonds involve two pairs, and triple bonds involve three pairs. The strength of the bond increases with the number of shared electron pairs, with triple bonds being the strongest.
Importance of Covalent Bonds
Covalent bonds play a crucial role in the formation of molecules and the interactions between atoms in many different substances. These bonds occur when two or more atoms share electrons in their outer shells, resulting in a stable configuration for both atoms. This sharing of electrons allows atoms to achieve a more stable state, similar to having a full outer shell like the noble gases. Covalent bonds are especially important in organic chemistry, as they are the basis for the formation of complex organic compounds.
One of the main reasons covalent bonds are important is that they dictate the properties and behavior of substances. The type and strength of covalent bonds between atoms determine factors such as the melting point, boiling point, solubility, and reactivity of a substance. The number and arrangement of shared electrons also influence the shape and polarity of molecules, which in turn affects their chemical and physical properties.
In addition to determining the properties of substances, covalent bonds also contribute to the stability of molecules. By sharing electrons, atoms can fill their valence shells and achieve a more stable state. This stability is crucial for the functioning of biological molecules such as DNA and proteins. Covalent bonds also provide structural integrity to solid materials, such as diamond, which is composed entirely of carbon atoms bonded together in a covalent network.
Examples of Covalent Bonds
- Water (H2O) is held together by covalent bonds between the hydrogen and oxygen atoms.
- Methane (CH4) is a covalent compound consisting of carbon bonded to four hydrogen atoms.
- Nitrogen gas (N2) is composed of two nitrogen atoms held together by a triple covalent bond.
- Proteins are long chains of amino acids linked together by covalent peptide bonds.
- Carbon dioxide (CO2) has two double covalent bonds between carbon and oxygen atoms.
Overall, covalent bonds are essential for the stability, properties, and functionality of molecules and substances. They allow atoms to achieve a more stable electron configuration, leading to the formation of diverse compounds with a wide range of properties. Understanding covalent bonding is fundamental to chemistry and is crucial for many applications in fields such as materials science, pharmaceuticals, and biochemistry.
How to Use Gizmos Student Exploration Covalent Bonds
The Gizmos Student Exploration Covalent Bonds is an interactive online tool that allows students to explore and understand the concept of covalent bonds. This tool provides a hands-on learning experience for students, allowing them to manipulate atoms and molecules to observe the formation of covalent bonds.
To use the Gizmos Student Exploration Covalent Bonds, begin by selecting an element from the Periodic Table. This will be the central atom in your molecule. Then, choose another element to bond with the central atom. The tool will automatically show the atomic symbols and their respective valence electrons.
To form a covalent bond, students can drag and drop the valence electrons of the two atoms. As they do so, they will observe the formation of a shared electron pair, representing the covalent bond. The tool also provides information about the type of bond formed, whether it is a single, double, or triple covalent bond.
As students explore different combinations of elements and the formation of covalent bonds, they can observe how the properties of the molecules change. The Gizmo also allows them to visualize the molecular shape and bond angles, helping them understand the three-dimensional structure of molecules.
Overall, the Gizmos Student Exploration Covalent Bonds is a valuable tool for teaching and learning about covalent bonds. It provides an interactive and engaging learning experience, allowing students to manipulate atoms and molecules to observe and understand the formation of covalent bonds.
FAQs about Covalent Bonds
What is a covalent bond?
A covalent bond is a type of chemical bond formed between atoms when they share electrons. In this bond, two or more atoms come together and share their valence electrons to achieve a stable electron configuration. Covalent bonds are typically formed between nonmetallic elements.
How is a covalent bond different from an ionic bond?
Unlike ionic bonds, which involve a transfer of electrons from one atom to another, covalent bonds involve the sharing of electrons between atoms. In covalent bonds, electrons are shared in a way that both atoms have access to a complete outer electron shell, resulting in a more stable arrangement.
What determines the strength of a covalent bond?
The strength of a covalent bond depends on the electronegativity difference between the atoms involved. Electronegativity is a measure of an atom’s ability to attract shared electrons. If the electronegativity difference is large, the bond is polar covalent and the electrons are shared unequally. If the electronegativity difference is small or zero, the bond is nonpolar covalent and the electrons are shared equally.
Can covalent bonds form between different elements?
Yes, covalent bonds can form between different elements. The ability of atoms to form covalent bonds depends on their valence electron configuration and the number of electrons needed to achieve a stable configuration. Different elements have different electronegativities, which can influence the nature of the covalent bond.
What are some examples of covalent compounds?
Some examples of covalent compounds include water (H2O), methane (CH4), ammonia (NH3), and carbon dioxide (CO2). These compounds are formed through the sharing of electrons between atoms of different elements, resulting in a stable molecule.
Can you break a covalent bond?
Yes, covalent bonds can be broken through chemical reactions. This can occur by adding energy to the system, such as through the input of heat or the application of an external force. Breaking a covalent bond results in the separation of the shared electrons and the formation of new chemical species.