Understanding the thermal properties of substances is crucial in many scientific and engineering fields, especially when it comes to heating and cooling processes. One tool that helps us visualize these properties is the heating curve, which shows the relationship between temperature and heat added or removed from a substance during a phase change.
The heating curve worksheet is a valuable educational resource that provides students with an opportunity to practice using heating curves to analyze and interpret data. It allows students to apply their knowledge of thermodynamics and phase changes while solving problems related to heating and cooling processes.
With the heating curve worksheet answer key, students can check their solutions and verify their understanding of the concepts presented. The answer key provides step-by-step explanations for each problem, ensuring that students grasp the underlying principles and calculations involved in determining the heat transferred during phase changes.
Furthermore, the heating curve worksheet answer key allows teachers to assess students’ progress and identify any areas of difficulty. It serves as a valuable teaching tool, helping educators tailor their instruction and address specific areas of misconception or confusion.
In conclusion, the heating curve worksheet answer key plays a vital role in enhancing students’ understanding of thermal properties and phase changes. It promotes active learning, enables self-assessment, and supports effective teaching strategies. By engaging with the worksheet and its answer key, students can deepen their knowledge of thermodynamics and develop their problem-solving skills.
Heating Curve Worksheet Answer Key
In the study of thermodynamics, a heating curve is a graphical representation of the temperature change of a substance as it is heated. This curve can provide valuable information about the phase transitions and energy changes that occur during the heating process. A heating curve worksheet is a tool used by students to practice analyzing and interpreting these curves.
The answer key to a heating curve worksheet provides the correct answers or solutions to the questions and problems posed in the worksheet. It serves as a reference for students to check their work and ensure that they have correctly understood and applied the concepts related to heating curves.
In the answer key, you will find the numerical values for the specific heat capacity, latent heat of fusion, and latent heat of vaporization for the substance being heated. These values are necessary for calculating the total energy absorbed or released during each phase transition. The key may also include explanations or step-by-step solutions for more complex problems, such as determining the temperature of the substance at a specific point in the heating curve.
It is important to use the answer key responsibly and not rely solely on it for learning. The purpose of the heating curve worksheet is to enhance understanding and problem-solving skills. Students should attempt the problems on their own first and then use the answer key as a tool for self-assessment and clarification. Discussing the solutions with a teacher or classmates can also be beneficial for further learning and understanding.
The heating curve worksheet answer key is a valuable resource for students studying thermodynamics. It helps them practice analyzing and interpreting heating curves and ensures that they have a solid understanding of the concepts and calculations involved. By using the answer key responsibly and engaging in discussion and reflection, students can gain a deeper understanding of thermodynamics and improve their problem-solving skills.
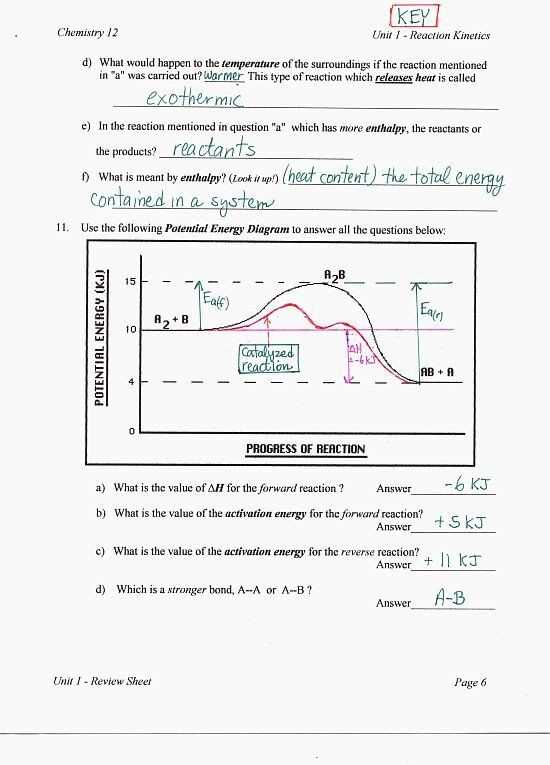
Understanding the Heating Curve Graph
The heating curve graph is a visual representation of how the temperature of a substance changes as heat is applied to it. It provides valuable information about the different phases of matter and the energy required to transition between them. By analyzing the heating curve graph, scientists and engineers can better understand the behavior of substances and design effective heating and cooling systems.
The x-axis of the heating curve graph represents time, while the y-axis represents temperature. The graph typically consists of multiple linear segments and plateaus, each corresponding to a different phase of matter. The gradual increase in temperature during the heating process is represented by the linear segments, while the plateaus indicate phase transitions where the energy supplied is used to change the substance’s structure rather than raise its temperature.
One key feature of the heating curve graph is the slope of the linear segments. The slope represents the rate at which the substance’s temperature is changing. Steeper slopes indicate faster heating rates, while shallower slopes indicate slower rates. This information is essential for determining the time required to heat or cool a substance and to design heating systems that can achieve desired temperature changes efficiently.
Another crucial aspect of the heating curve graph is the plateaus. During these plateaus, the substance is undergoing a phase transition, such as melting or vaporization. The temperature remains constant despite the continuous addition of heat because the energy is being used to change the substance’s state rather than increase its temperature. The length of these plateaus can provide insights into the substance’s heat capacity and latent heat, which are important for designing cooling systems and ensuring efficient energy transfer.
In conclusion, the heating curve graph is a powerful tool for understanding the behavior of substances during heating and cooling processes. By analyzing the slope of the linear segments and the length of the plateaus, scientists and engineers can gain insights into the substance’s properties and design effective heating and cooling systems. It is an essential tool in fields such as thermodynamics, materials science, and engineering, enabling advancements in various industries and technologies.
The Phases of Heating Curves
Heating curves represent the changes in temperature and phase that a substance undergoes when heat is added to it. They are often used to analyze and understand the behavior of materials during heating processes. The phases of a heating curve are characterized by distinct temperature ranges and changes in the substance’s physical state.
First, we have the solid phase, where the substance is in a solid state. During this phase, the temperature remains constant as heat energy is used to overcome the intermolecular forces holding the particles together. This portion of the curve is called the plateau or the melting point, and it represents the transition from solid to liquid state. Once all the solid is transformed into liquid, the temperature starts to rise again.
The next phase is the liquid phase, where the substance is in a liquid state. As heat is continuously added, the temperature gradually increases, until it reaches a maximum point called the boiling point. At this point, the liquid starts to vaporize and transform into a gas. The temperature again remains constant during this phase, as the heat energy is used to overcome the intermolecular forces and break the liquid’s cohesive structure.
Finally, we have the gas phase, where the substance is in a gaseous state. In this phase, the temperature continues to rise with the addition of heat energy. The heating curve ends when the substance reaches its highest temperature. At this point, no more phase changes occur, and the substance remains in the gaseous state until further heating occurs.
In conclusion, heating curves provide a visual representation of the different phases and temperature changes that occur when a substance is heated. Understanding the phases of a heating curve is crucial in various fields, including chemistry, physics, and materials science, as it allows scientists to predict and control the behavior of materials during heating processes.
Interpreting Temperature and Time Relationships
The relationship between temperature and time is crucial in understanding heating curves. A heating curve represents the changes in temperature as a substance is heated or cooled, usually shown on a graph. By interpreting this relationship, we can gain insights into the properties and behavior of the substance.
When a substance is heated, its temperature typically increases over time. The rate of temperature increase depends on several factors, including the amount of heat applied, the specific heat capacity of the substance, and its mass. This relationship can be represented as a straight line on a heating curve, with the temperature increasing linearly with time.
As the substance reaches its boiling point or melting point, the temperature remains constant for a period of time. This plateau-like region is known as the phase change, where the substance undergoes a physical change without a change in temperature. During this phase change, the heat supplied is used to break the intermolecular forces holding the substance together, rather than increasing the temperature.
Once the phase change is complete, the temperature resumes increasing again, following the same linear relationship with time as before. By analyzing the slope of the heating curve during different sections, we can determine the specific heat capacity of the substance and its energy requirements for phase changes.
Understanding the temperature and time relationship in a heating curve is crucial for a variety of applications, including cooking, material science, and industrial processes. It allows us to determine the optimal heating and cooling rates for different substances, as well as predict the behavior of materials under different conditions.
Calculating Heat Transfer
When it comes to understanding heat transfer, one of the key factors to consider is the amount of heat that is transferred. This can be determined by calculating the heat transfer equation, which is Q = mcΔT. In this equation, Q represents the amount of heat transferred, m represents the mass of the substance being heated or cooled, c represents the specific heat capacity of the substance, and ΔT represents the change in temperature.
To calculate the heat transfer, you need to know the values of the mass, specific heat capacity, and change in temperature. The mass can be measured in grams or kilograms, and the specific heat capacity is a characteristic property of the substance being heated or cooled. The change in temperature is determined by subtracting the initial temperature from the final temperature.
In some cases, you may also need to consider the heat of fusion or heat of vaporization when calculating heat transfer during a phase change. The heat of fusion is the amount of heat required to change a substance from a solid to a liquid, while the heat of vaporization is the amount of heat required to change a substance from a liquid to a gas. These values can be used in addition to the specific heat capacity to determine the total amount of heat transferred during a phase change.
Overall, calculating heat transfer is an important aspect of understanding how energy is transferred in a system. By using the heat transfer equation and considering factors such as mass, specific heat capacity, and changes in temperature, you can determine the amount of heat that is transferred during a heating or cooling process.
Using the Heating Curve Worksheet
The Heating Curve Worksheet is a helpful tool for students to understand and analyze the changes in temperature and energy during a heating process. This worksheet provides a visual representation of the heating curve and allows students to answer questions and make observations based on the provided data.
One key aspect of the Heating Curve Worksheet is the graph that displays the temperature changes over time. This graph is divided into different segments that correspond to the different phases of matter, such as solid, liquid, and gas. By examining the graph, students can identify the specific temperature ranges where phase changes occur, such as melting or boiling points.
Another important component of the worksheet is the set of questions that accompany the graph. These questions prompt students to apply their knowledge of thermodynamics and phase changes to interpret the data. For example, students may be asked to determine the energy transfer during specific phases or calculate the temperature change during a phase change.
The Heating Curve Worksheet encourages critical thinking and problem-solving skills. Students must analyze the graph, interpret the data, and apply their understanding of thermodynamics concepts to answer the questions accurately. By using this worksheet, students can deepen their understanding of heating processes and enhance their ability to analyze and interpret data in a scientific context.
Common Mistakes and Troubleshooting
When working with a heating curve worksheet, it is common to encounter some mistakes or encounter difficulties. Here are some of the most common mistakes and troubleshooting tips to help you navigate through them.
Not Understanding the Concept
One common mistake is not fully understanding the concept of a heating curve. It is important to grasp the idea that the curve represents the changes in temperature and state of matter as a substance is heated or cooled.
One way to troubleshoot this issue is to review the basics. Make sure you understand the difference between temperature and heat, as well as the different phases of matter and the changes that occur during heating or cooling.
Misinterpreting the Graph
Another common mistake is misinterpreting the information presented on the graph. It is important to read the graph carefully and understand what each part of the curve represents.
To troubleshoot this issue, take your time to analyze the graph and read the labels and axis carefully. Pay attention to any changes in slope or plateaus that indicate state changes or changes in the heating or cooling rate.
Calculation Errors
Calculations are a crucial part of working with a heating curve worksheet, and mistakes in calculations can lead to incorrect answers. Common calculation errors include mixing up units, failing to convert between units, or making arithmetic mistakes.
To troubleshoot this issue, double-check your calculations and make sure you are using the correct units. If you are unsure about a specific calculation, review the relevant equations and formulas, or ask for clarification from your teacher or classmates.
Lack of Practice
One final mistake is not practicing enough with heating curve worksheets. Like any other skill, understanding and interpreting heating curves requires practice and repetition. Without enough practice, errors and misunderstandings are more likely to occur.
To troubleshoot this issue, make sure you have sufficient practice problems to work on. Try to work through them on your own and seek feedback from your teacher or classmates to identify any areas for improvement.