
The history of atoms is a fascinating journey that spans thousands of years. It is a story of human curiosity, scientific discovery, and the quest to understand the fundamental building blocks of matter. From ancient Greek philosophers to modern-day scientists, each generation has contributed to our understanding of atoms and their properties. In this article, we will explore the history of atoms and provide answers to common questions about their structure, behavior, and significance in the world around us.
Let us begin our exploration by delving into the origins of atomic theory. The concept of atoms dates back to ancient times, with philosophers such as Democritus proposing that matter is composed of indivisible particles. However, it was not until the 19th century that scientific evidence began to support the existence of atoms. Pioneering work by chemists John Dalton and Antoine Lavoisier laid the foundation for modern atomic theory, providing experimental proof for the existence of atoms and their role in chemical reactions.
As our understanding of atoms grew, so did our curiosity about their internal structure. One of the most important breakthroughs in this regard came from the experiments of Ernest Rutherford in the early 20th century. Rutherford’s gold foil experiment revealed the presence of a tiny, positively charged nucleus at the center of an atom, surrounded by a cloud of negatively charged electrons. This discovery revolutionized our understanding of atomic structure, leading to the development of the modern atomic model.
Today, we have a wealth of knowledge about atoms and their properties. Scientists have discovered subatomic particles such as protons, neutrons, and electrons, each playing a crucial role in the behavior and characteristics of atoms. The field of quantum mechanics has further deepened our understanding of atomic behavior, providing insights into the wave-like nature of electrons and the probabilistic nature of their position within an atom.
In conclusion, the history of atoms is a tale of human curiosity and scientific progress. From the ancient Greeks to modern-day researchers, we have come a long way in our understanding of atoms and their properties. By studying the history of atomic theory, we can gain a deeper appreciation for the scientific achievements that have shaped our understanding of the world at its most fundamental level.
History of Atom Worksheet Answers
The history of the atom is a fascinating journey that spans thousands of years. From the early Greek philosophers to modern scientists, humans have been trying to understand the fundamental building blocks of matter. This worksheet provides answers to questions about the key moments and figures in the history of the atom.
1. Who discovered the electron?
The electron was discovered by J.J. Thomson in 1897. Using a cathode ray tube, he observed a stream of negatively charged particles, which he called electrons. This discovery revolutionized our understanding of atomic structure and led to the development of the modern model of the atom.
2. What is Rutherford’s atomic model?
Rutherford’s atomic model, also known as the planetary model, proposed that atoms have a small, dense nucleus at the center, surrounded by orbiting electrons. This model was based on the results of the famous gold foil experiment, in which alpha particles were fired at a thin gold foil. The observation that some particles were deflected suggested the existence of a concentrated positive charge in the nucleus.
3. Who first described the concept of atomic theory?
The concept of atomic theory can be traced back to the ancient Greek philosopher Democritus. In the 5th century BC, Democritus proposed that matter is composed of tiny, indivisible particles called atoms. Although his theory lacked experimental evidence, it laid the groundwork for future scientists to explore and develop the field of atomic theory.
4. What did Niels Bohr contribute to the understanding of the atom?
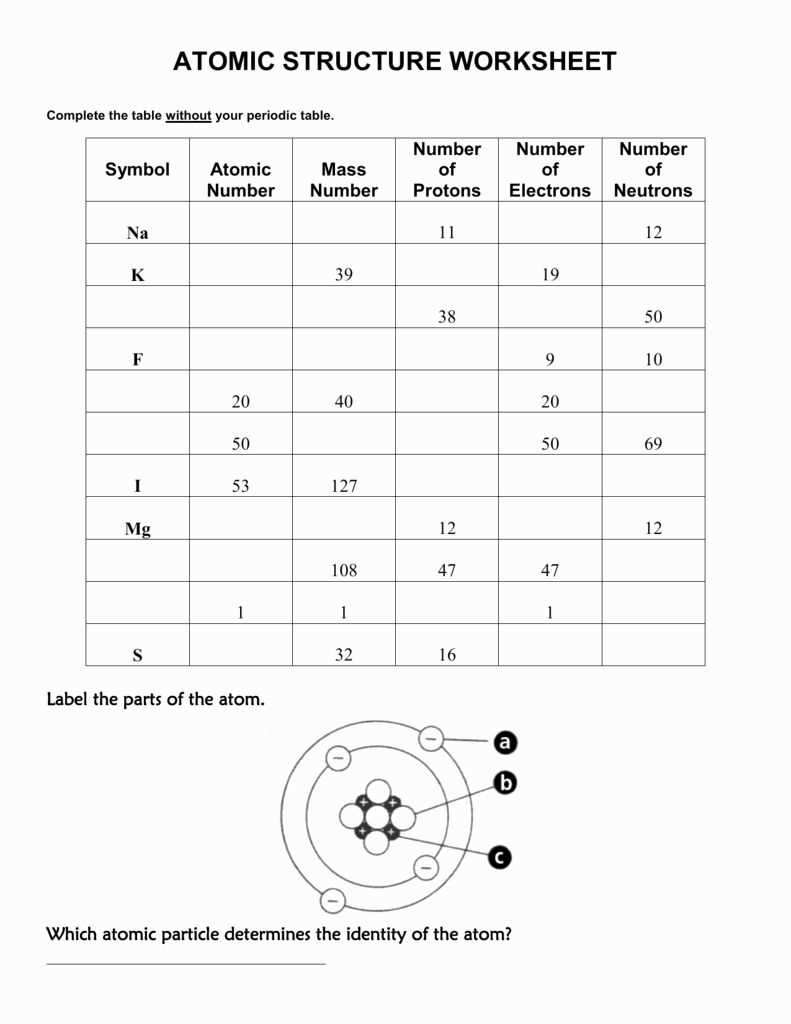
Niels Bohr made significant contributions to the understanding of the atom, particularly with his development of the Bohr model. This model introduced the idea that electrons exist in specific energy levels or shells around the nucleus. Additionally, Bohr’s model explained the phenomenon of atomic spectra, which is the unique pattern of light emitted or absorbed by an atom.
5. Who proposed the concept of the atomic nucleus?
Ernest Rutherford proposed the concept of the atomic nucleus in 1911. Through his gold foil experiment, he demonstrated that most of the mass of an atom is concentrated in a small, positively charged nucleus. This discovery challenged the prevailing model at the time, known as the plum pudding model, which suggested that the positive charge was evenly distributed throughout the atom.
Conclusion:
The history of the atom is a continuous process of discovery and innovation. From ancient philosophical speculations to modern experimental techniques, scientists have taken us on a journey to unravel the mysteries of the atom. The answers provided in this worksheet highlight some of the key milestones in this fascinating scientific endeavor.
Ancient Greek Philosophers’ Theories on Atoms
The ancient Greek philosophers were among the first to propose theories on the nature of atoms. These philosophers, such as Democritus and Leucippus, believed that everything in the universe was composed of tiny, indivisible particles called atoms. They theorized that these atoms were in constant motion and were the building blocks of all matter.
Democritus, specifically, proposed that atoms were made up of tiny, indestructible particles that differed in size, shape, and arrangement. He believed that these atoms combined and formed different substances by hooking onto each other. This theory of atomism was groundbreaking at the time and laid the foundation for future scientific discoveries.
Despite lacking the advanced technology and instruments that we have today, these ancient Greek philosophers were able to conceptualize the idea of atoms and develop theories that are still influential in modern science. Their understanding of atoms and their properties paved the way for further exploration and advancements in the field of chemistry and physics.
John Dalton’s Atomic Theory
John Dalton was an English chemist and physicist who lived from 1766 to 1844. He is widely known for his groundbreaking work in the field of atomic theory. Dalton’s atomic theory was based on several key principles that helped shape our understanding of the fundamental building blocks of matter.
1. Elements are composed of tiny particles called atoms. Dalton proposed that all elements are made up of small indivisible particles called atoms. These atoms are the fundamental units of matter and cannot be further broken down into simpler substances. Each element is characterized by a unique type of atom.
2. Atoms of the same element are identical. According to Dalton, all atoms of the same element are identical in terms of their size, mass, and chemical properties. This means that all atoms of oxygen, for example, are identical to each other and different from atoms of any other element.
3. Atoms can combine to form compounds. Dalton proposed that atoms can combine with each other in specific ways to form compounds. These compounds are formed when atoms of different elements combine in simple whole number ratios. For example, water is formed by the combination of two hydrogen atoms and one oxygen atom.
4. Chemical reactions involve rearrangement of atoms. According to Dalton’s theory, chemical reactions occur when atoms are rearranged to form new compounds. The atoms themselves remain unchanged in terms of their identities and properties, but they can be rearranged to form different substances.
5. Atoms are indivisible and indestructible. Dalton believed that atoms cannot be divided or destroyed in ordinary chemical reactions. They are the smallest units of matter that retain the properties of the element. This principle laid the foundation for our understanding of the conservation of mass in chemical reactions.
Overall, John Dalton’s atomic theory revolutionized our understanding of the nature of matter. His ideas provided a framework for studying and predicting the behavior of atoms and molecules, and his work laid the foundation for modern atomic theory.
J.J. Thomson’s Discovery of Electrons
One of the most groundbreaking discoveries in the field of atomic structure was made by J.J. Thomson in the late 19th century. Thomson conducted a series of experiments with cathode rays, which were streams of negatively charged particles that were emitted from the cathode in a vacuum tube. Through his experiments, Thomson was able to determine that these particles were a fundamental component of atoms and he named them electrons.
Thomson’s work revolutionized the understanding of atomic structure at the time. Prior to his discovery, atoms were believed to be indivisible, but Thomson’s experiments showed that they were composed of smaller particles. He proposed a model called the “plum pudding” model, in which the electrons were scattered throughout a positively charged cloud. This model provided the first visualization of atomic structure and laid the foundation for further research in the field.
To support his findings, Thomson performed calculations and measurements to determine the charge-to-mass ratio of electrons. His experiments showed that electrons had a very small mass compared to the overall mass of an atom, but carried a negative charge. This led to the development of the concept of atomic particles with different charges and masses, which eventually paved the way for the discovery of protons and neutrons.
Thomson’s discovery of electrons not only advanced the field of atomic theory, but also had significant implications in other areas of science and technology. The understanding of electrons and their behavior laid the foundation for the development of electronics and the field of quantum mechanics. Furthermore, Thomson’s work also opened up new possibilities for the study of atomic and molecular structure, which continue to be explored and expanded upon to this day.
Ernest Rutherford’s Gold Foil Experiment
Ernest Rutherford’s Gold Foil Experiment, conducted in 1909, played a crucial role in our understanding of the atom. The experiment aimed to investigate the structure of atoms and test the prevailing Plum Pudding model proposed by J.J. Thomson.
Rutherford and his team used a thin sheet of gold foil and directed a beam of alpha particles at it. Alpha particles are positively charged particles emitted by radioactive substances. According to the Plum Pudding model, the alpha particles were expected to pass straight through the gold foil with minimal deflection.
However, to everyone’s surprise, Rutherford observed that some of the alpha particles were deflected at large angles and even bounced back. This unexpected result led Rutherford to propose a new atomic model, known as the Nuclear Model or Rutherford Model.
The key findings from the Gold Foil Experiment were as follows:
- Most of the alpha particles passed through the gold foil with minimal deflection, indicating that atoms are mostly empty space.
- Some alpha particles were deflected at small angles, implying the presence of a concentrated positive charge in the atom.
- A small number of alpha particles were redirected back towards the source, suggesting the existence of a dense, positively charged nucleus at the center of the atom.
- The size of the nucleus was estimated to be much smaller than the overall size of the atom.
Rutherford’s Gold Foil Experiment revolutionized our understanding of the atom by disproving the Plum Pudding model and introducing the idea of the nucleus. It paved the way for further research and developments in atomic physics, ultimately leading to the modern understanding of atomic structure.
Niels Bohr’s Model of the Atom
One of the most influential models of the atom was developed by Danish physicist Niels Bohr in 1913. This model, known as the Bohr model or the planetary model, revolutionized our understanding of atomic structure and laid the foundation for modern quantum mechanics.
According to Bohr’s model, the atom is composed of a central nucleus containing positively charged protons and neutral neutrons, surrounded by negatively charged electrons in specific energy levels or shells. These energy levels are represented by discrete circular orbits around the nucleus, similar to the planets orbiting the sun.
The electrons in Bohr’s model can only exist in certain energy levels, or shells, and they can transition between these levels by absorbing or emitting energy in the form of photons. When an electron absorbs energy, it moves to a higher energy level or shell. Conversely, when an electron emits energy, it moves to a lower energy level. This energy transition is responsible for the emission and absorption spectra observed in atoms.
In addition to explaining the stability of atoms, Bohr’s model also provided an understanding of the periodic table and the chemical behavior of elements. The number of electrons in the outermost shell determines the reactivity and chemical properties of an atom. This concept is crucial in understanding chemical bonding and the formation of compounds.
Although Bohr’s model has been succeeded by more complex quantum mechanical models, it remains a fundamental part of atomic theory and continues to be taught in introductory chemistry and physics classes. It represents a significant milestone in our understanding of the atom and has paved the way for further discoveries in quantum physics.
The Discovery of the Neutron by James Chadwick
James Chadwick’s discovery of the neutron in 1932 was a groundbreaking achievement in the field of atomic theory. Building upon the work of other scientists, including Ernest Rutherford, Chadwick was able to provide evidence for the existence of a neutral atomic particle, which he named the neutron.
Chadwick’s discovery came as a result of his experiments with the newly discovered phenomenon of radioactivity. He noticed that when certain elements, such as beryllium, were bombarded with alpha particles, a new type of radiation was emitted. Unlike alpha and beta particles, this radiation was not deflected by electric or magnetic fields and had no charge.
To further investigate this radiation, Chadwick designed a series of experiments using thin sheets of various elements. He observed that when the radiation passed through these sheets, it caused the emission of protons. This led him to conclude that the radiation consisted of neutral particles, which he called neutrons.
This discovery had significant implications for our understanding of the atom. Prior to Chadwick’s work, it was believed that the atomic nucleus consisted only of positively charged protons. The discovery of the neutron provided an explanation for the stability of the nucleus, as the neutrons balanced out the positive charge of the protons and prevented them from repelling each other.
Chadwick’s discovery of the neutron paved the way for further advancements in atomic theory and laid the foundation for the development of nuclear energy and technology. His contributions to the field earned him the Nobel Prize in Physics in 1935 and solidified his place as one of the most influential scientists of the 20th century.