
The periodic table is a systematic arrangement of chemical elements that provides valuable information about their properties and behavior. It is organized in a way that allows scientists to easily understand and study the elements based on their atomic number, electron configuration, and recurring patterns in their chemical properties.
One way to understand the periodic table is to look at it as a giant grid divided into blocks. Each block represents a different group or category of elements, such as metals, non-metals, and metalloids. The elements are arranged in order of increasing atomic number, from left to right and top to bottom. This arrangement helps scientists identify trends and relationships between elements.
The periodic table is also divided into periods, which are horizontal rows, and groups, which are vertical columns. Each period corresponds to a different energy level or shell in the electron configuration of the elements. The groups, on the other hand, represent elements with similar chemical properties and outer electron configurations. This organization allows scientists to easily predict the behavior of elements and their ability to form compounds.
By studying the periodic table, scientists can understand the relationships between elements and use this knowledge to make predictions and develop new materials. It is a valuable tool that continues to be expanded and updated as new elements are discovered and our understanding of the elements deepens. By using worksheets, students can practice their knowledge of the periodic table and improve their understanding of the elements and their properties.
Understanding the periodic table
The periodic table is a powerful tool that helps chemists organize and understand the elements. It provides a systematic way of arranging elements based on their atomic structure, allowing scientists to predict and explain various chemical properties and behaviors.
Organization: The periodic table is organized into rows called periods and columns called groups. Each element is represented by a symbol and is placed in a specific location on the table based on its atomic number. The atomic number indicates the number of protons in an atom’s nucleus and determines its unique chemical identity.
Periods: The elements in each period are arranged in order of increasing atomic number. As you move from left to right across a period, you’ll notice a gradual increase in atomic size and a progression of changing chemical properties. This is due to the increasing number of electrons in the outermost energy level, which affects how elements interact chemically.
Groups: The elements in each group share similar chemical properties. The groups are numbered from 1 to 18, and they can be further divided into main groups and transition metals. Elements in Group 1, for example, are known as alkali metals and are highly reactive. Elements in Group 17, on the other hand, are known as halogens and are highly reactive nonmetals.
Periodic trends: The pattern of elements on the periodic table reveals various trends in their properties. For example, as you move down a group, elements tend to increase in size due to the addition of energy levels. Electronegativity, the ability of an atom to attract electrons, tends to decrease as you move down a group. These trends can help scientists make predictions about how elements will interact in chemical reactions.
Applications: The periodic table is not just a theoretical tool for chemists; it has practical applications as well. Its organized structure allows scientists to identify and study unknown elements, predict the behavior of new compounds, and design materials with specific properties. The periodic table is also used to classify elements into different categories, such as metals, nonmetals, and metalloids.
In conclusion, understanding the periodic table is essential for anyone studying chemistry. It provides a framework for organizing and explaining the properties of elements, enabling scientists to make predictions and discoveries that contribute to our understanding of the natural world.
What is the periodic table and why is it important?

The periodic table is a tabular arrangement of chemical elements, organized based on their atomic number, electron configuration, and recurring chemical properties. It is a crucial tool in chemistry and provides essential information about the properties and relationships of elements.
The organization of elements
The periodic table is divided into groups and periods. Groups are vertical columns that share similar chemical properties, while periods are horizontal rows that represent the successive energy levels of an atom’s electron shells. This organization allows scientists to identify trends and patterns in element properties as well as predict properties of yet-to-be-discovered elements.
Element information
The periodic table provides detailed information about each element. For example, it displays the atomic number, symbol, atomic mass, electron configuration, and the number of valence electrons for each element. This information is crucial for understanding an element’s behavior, reactivity, and its ability to form compounds.
Predicting element properties
By studying the periodic table, scientists can make predictions about the properties of elements. For example, elements in the same group tend to have similar chemical behaviors and react similarly with other elements. Additionally, the periodic table helps in identifying periodic trends such as atomic radius, ionization energy, electronegativity, and chemical reactivity.
Applications in research and industry
The periodic table is essential in various fields of science, including chemistry, physics, and materials science. It helps researchers in understanding and experimenting with chemical reactions, discovering new elements, and developing new materials with specific properties. It also plays a crucial role in industrial processes, such as determining the suitability of elements for specific applications and designing efficient chemical reactions.
Overall, the periodic table is an indispensable tool for scientists and researchers as it provides a systematic and organized way to understand the properties and relationships of elements. It is a fundamental cornerstone of chemistry and enables advancements in various scientific fields.
Organization of the periodic table
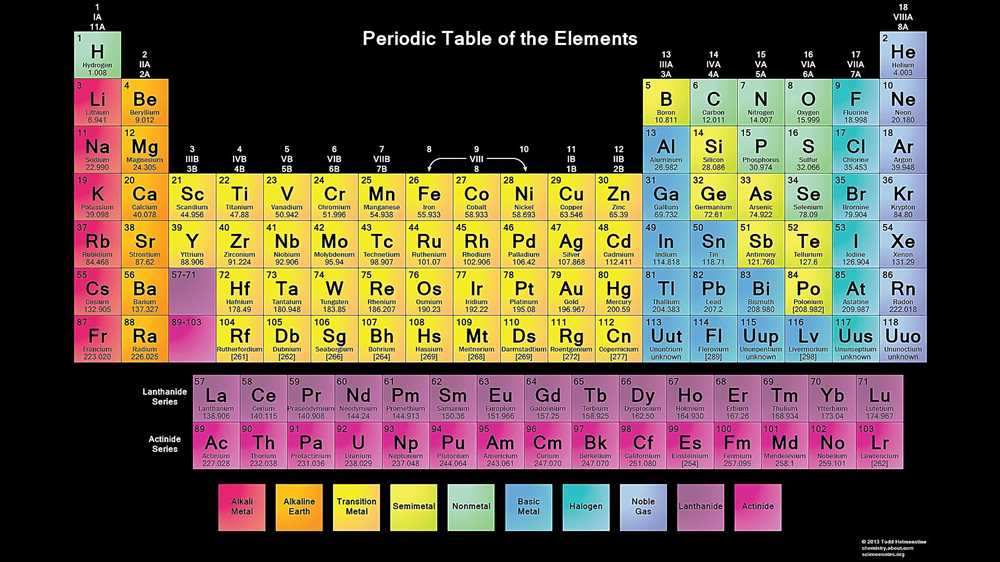
The periodic table is a systematic arrangement of chemical elements in order of their atomic number, electron configuration, and recurring chemical properties. It is a valuable tool for scientists to understand and predict the properties and behavior of different elements.
The periodic table is organized into periods (rows) and groups (columns). Each element is assigned a unique symbol and is placed in a specific location based on its atomic number. The atomic number represents the number of protons in an atom’s nucleus, which determines its chemical identity.
The periodic table is divided into several distinct blocks: s-block, p-block, d-block, and f-block. These blocks correspond to different types of electron configurations. Elements in the s-block have their valence electrons in the s-orbital, p-block elements have their valence electrons in the p-orbital, d-block elements have their valence electrons in the d-orbital, and f-block elements have their valence electrons in the f-orbital.
The elements in each group of the periodic table share similar properties due to their similar electron configurations. For example, the elements in Group 1 (alkali metals) have one valence electron and exhibit similar reactivity. The elements in Group 18 (noble gases) have full outer electron shells and are generally unreactive.
In addition to organizing elements based on their electron configurations, the periodic table also provides valuable information about the elements’ atomic mass, symbols, and names. It is a comprehensive reference tool that helps scientists explore and understand the diverse world of chemical elements.
How is the periodic table organized?

The periodic table is a systematic arrangement of the chemical elements. It is organized based on the atomic number, which represents the number of protons in the nucleus of an atom. The elements are arranged in order of increasing atomic number from left to right and top to bottom. Each element is assigned a unique symbol, typically consisting of one or two letters, to represent it.
The periodic table is divided into several sections and groups. The main sections are the s-block, p-block, d-block, and f-block. The elements in each section have similar properties due to their electron configurations. The groups, which are vertical columns, are numbered from 1 to 18. Elements in the same group have similar chemical properties, as they have the same number of valence electrons.
The periodic table also contains periods, which are the horizontal rows. There are currently seven periods in the periodic table. The elements in each period have the same number of electron shells. As you move across a period, the atomic number increases, and the elements become less metallic in nature.
Additionally, the periodic table is color-coded to indicate different categories of elements. The main categories include metals, nonmetals, and metalloids. Metals are typically located on the left side of the periodic table, while nonmetals are found on the right side. Metalloids, which have properties of both metals and nonmetals, are situated along the zigzag line that separates the two categories.
In conclusion, the periodic table is organized based on the atomic number of elements, with similar elements placed in the same groups and periods. This systematic arrangement allows scientists to easily access and understand the properties and behaviors of various elements.
The Periodic Table Structure
The periodic table is a tabular arrangement of chemical elements, organized based on their atomic numbers, electron configurations, and recurring chemical properties. This arrangement enables scientists to study and understand the properties and behavior of different elements, as well as predict and analyze their reactions and compounds.
The periodic table is divided into periods (horizontal rows) and groups (vertical columns). Each period represents a different electron shell, while each group shares similar outer electron configurations and exhibits similar chemical behaviors. The table is further organized into blocks, namely s-block, p-block, d-block, and f-block, based on the type of subshell being filled in each period.
The s-block consists of groups 1 and 2, which include the alkali metals and alkaline earth metals, respectively. These elements have valence electrons in the s-subshell and display highly reactive properties. The p-block comprises groups 13 to 18, which include elements such as the halogens and noble gases. These elements have valence electrons in the p-subshell and exhibit a wide range of properties.
The d-block, also known as the transition metals, fills the d-subshell in each period. These elements have unique electronic configurations and display characteristic properties, such as variable oxidation states and catalytic activities. Finally, the f-block consists of the lanthanides and actinides, which fill the f-subshell and are often referred to as the rare earth elements. These elements have complex electronic structures and exhibit unique magnetic and optical properties.
What are the different elements of the periodic table?
The periodic table is made up of different elements that are classified based on their chemical properties. There are 118 known elements, each with its own unique characteristics and atomic structure. These elements are arranged in the periodic table according to their atomic number, which represents the number of protons in their nucleus.
Elements in the periodic table can be further categorized into several groups, including metals, nonmetals, and metalloids. Metals, such as iron and copper, are typically shiny, malleable, and good conductors of heat and electricity. Nonmetals, like oxygen and sulfur, are generally gases or brittle solids with poor conductivity. Metalloids, such as silicon and arsenic, possess properties that are intermediate between metals and nonmetals.
- Alkali metals: These elements, including lithium and sodium, are highly reactive and usually exist in compounds rather than their pure form.
- Alkaline earth metals: Elements like calcium and magnesium belong to this group and are less reactive than alkali metals.
- Transition metals: This group includes familiar elements like iron, copper, and gold, known for their varied oxidation states and ability to form colorful compounds.
- Halogens: Fluorine, chlorine, and iodine are examples of halogens, highly reactive nonmetals that often form compounds with alkali and alkaline earth metals.
- Noble gases: This group contains inert gases like helium and neon, which have full valence electron shells and are chemically unreactive.
In addition to these groups, there are also rare earth elements, lanthanides, and actinides that occupy specific sections of the periodic table. These elements have unique properties and are often used in various technological applications.
Atomic Number and Atomic Mass
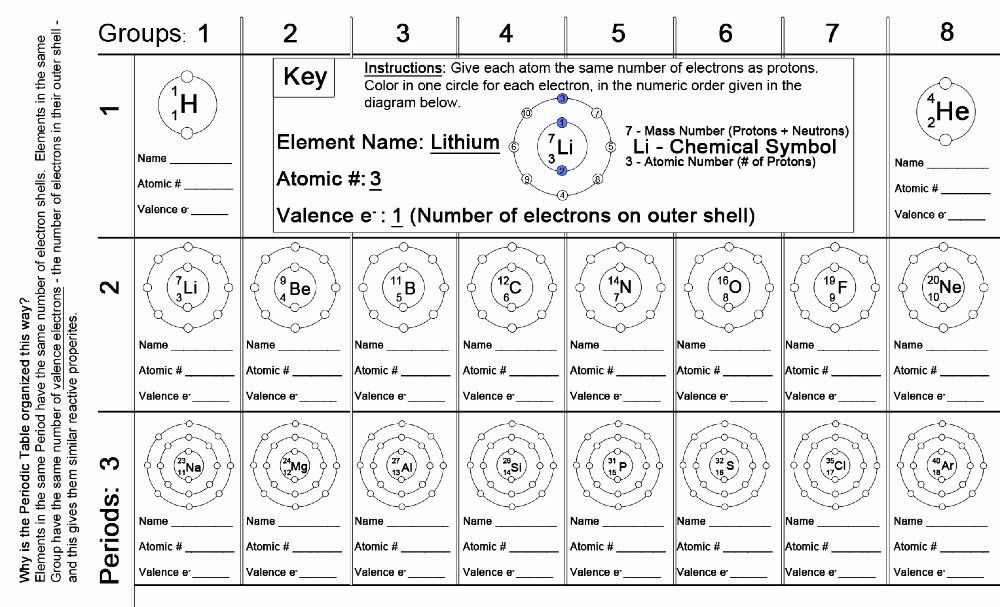
The periodic table is a systematic arrangement of chemical elements based on their atomic number and atomic mass. These two properties play a crucial role in understanding the organization of the elements in the table.
Atomic number is the number of protons found in the nucleus of an atom. It uniquely identifies an element and determines its position in the periodic table. The atomic number increases from left to right and from top to bottom in the periodic table. It represents the number of electrons as well, in a neutral atom.
Atomic mass, also known as atomic weight, is the average mass of all the isotopes of an element. It takes into account the relative abundance of each isotope and their individual masses. Atomic mass is represented in atomic mass units (amu) and is usually close to the whole number atomic number. The atomic mass increases from left to right and from top to bottom.
The arrangement of elements in the periodic table is based on these properties. Each element is organized in order of increasing atomic number and is placed in a specific group and period. Elements within the same group have similar chemical properties, while elements within the same period have similar electron configurations.
The periodic table provides a valuable tool for chemists and scientists to understand the relationships and trends among the elements. It allows for easy identification of elements and their properties, aiding in the study and prediction of chemical reactions and behavior.