
In the study of chemistry, understanding the properties and behaviors of acids and bases is crucial. Acids and bases are two fundamental types of compounds that have contrasting characteristics. Acids, for instance, are substances that can donate hydrogen ions (H+) and have a sour taste. On the other hand, bases are substances that can accept hydrogen ions and have a bitter taste. The concepts of acids and bases are often taught through worksheets, which allow students to practice identifying and categorizing different compounds.
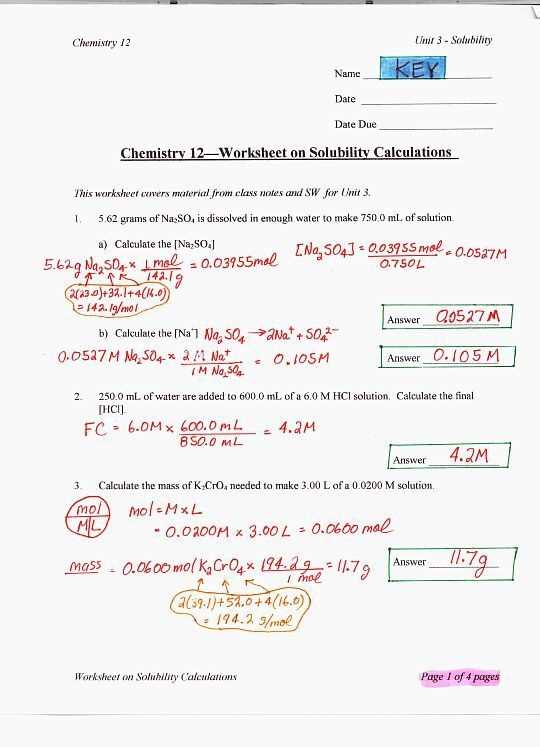
An acids and bases worksheet answer key is a valuable tool that provides the correct answers for a worksheet on acids and bases. This answer key allows students to check their work and ensure that they have correctly identified and categorized the compounds. By comparing their answers to the answer key, students can learn from their mistakes and gain a better understanding of acids and bases.
The acids and bases worksheet answer key typically includes a list of compounds and their corresponding classifications as acids or bases. It may also provide explanations or descriptions of why certain compounds are categorized as acids or bases. This additional information helps students grasp the underlying principles and concepts related to acids and bases.
Using an acids and bases worksheet answer key can enhance the learning experience by giving students immediate feedback and promoting self-assessment. It allows students to take ownership of their learning and become actively engaged in the subject matter. With a comprehensive answer key, students can become more confident in their understanding of acids and bases and apply their knowledge to solve more complex problems.
What are Acids?
Acids are chemical substances that have the ability to donate protons or accept electrons in a chemical reaction. They are characterized by their sour taste, ability to turn blue litmus paper red, and their corrosive nature. Acids can be found in various forms, including liquids, solids, and gases, and they play a crucial role in many biological, industrial, and environmental processes.
Acids are classified based on their strength, with strong acids completely dissociating in water to produce hydrogen ions (H+), while weak acids only partially dissociate. Examples of strong acids include hydrochloric acid (HCl) and sulfuric acid (H2SO4), while examples of weak acids include acetic acid (CH3COOH) and carbonic acid (H2CO3).
Acids have a lower pH value, typically ranging from 0 to 7, with 0 being the strongest and most acidic. They have the ability to react with bases to form salts and water in a chemical reaction known as neutralization. Some common acids include vinegar (acetic acid), lemon juice (citric acid), and stomach acid (hydrochloric acid).
Acids have a wide range of applications in everyday life. They are used in cleaning products, food preservation, metal etching, and as catalysts in chemical reactions. They are also key components in the functioning of the human body, playing roles in digestion and metabolism. Understanding acids and their properties is essential in many scientific disciplines, including chemistry, biology, and environmental science.
Common Properties of Acids
Acids are a class of compounds that have several properties in common. These properties help to distinguish acids from other substances. Here are some common properties of acids:
- Sour taste: One of the most well-known properties of acids is their sour taste. Substances like lemon juice and vinegar are acidic and have a distinct sour flavor.
- pH less than 7: Acids are characterized by having a pH less than 7 on the pH scale. The pH scale measures the acidity or basicity of a substance, with values below 7 indicating acidity.
- Ability to react with metals: Acids have the ability to react with certain metals, producing hydrogen gas and a salt. This reaction is commonly observed when acids come into contact with metals like zinc or magnesium.
- Corrosive properties: Acids are corrosive substances, meaning they can damage or dissolve other materials. This property is often used in industries such as cleaning, where acids are used to remove stains and build-up.
- Turns blue litmus paper red: Acids can be identified by their ability to turn blue litmus paper to red. Litmus paper is an indicator that changes color based on the acidity or basicity of a substance.
These common properties help to identify and classify substances as acids. It is important to note that not all acids exhibit all of these properties, but the presence of one or more of these characteristics can indicate the presence of an acid.
How Do Acids React with Bases?
Acids and bases are two types of chemical substances that can react with each other to form new products. The reaction between an acid and a base is called neutralization. During this reaction, the hydrogen ions (H+) from the acid combine with the hydroxide ions (OH-) from the base to form water (H2O). At the same time, the remaining ions from the acid and the base combine to form a salt.
One key property of acids and bases is their pH level. Acids have a pH less than 7, while bases have a pH greater than 7. When an acid and a base react, they tend to neutralize each other and form a solution with a pH close to 7, which is considered neutral. This is why neutralization reactions are often used to remove the acidic or basic properties of a solution.
In terms of their chemical formulas, acids are typically compounds that release hydrogen ions (H+) when dissolved in water. Examples of common acids include hydrochloric acid (HCl) and sulfuric acid (H2SO4). On the other hand, bases are compounds that release hydroxide ions (OH-) when dissolved in water. Examples of bases include sodium hydroxide (NaOH) and potassium hydroxide (KOH).
The reaction between acids and bases can be summarized by the following chemical equation: acid + base → salt + water. For example, when hydrochloric acid (HCl) reacts with sodium hydroxide (NaOH), they form sodium chloride (NaCl) and water (H2O). This reaction can be represented as HCl + NaOH → NaCl + H2O.
In summary, acids and bases react with each other through a neutralization reaction, where the hydrogen ions from the acid combine with the hydroxide ions from the base to form water. The remaining ions combine to form a salt. This reaction helps neutralize the acidic or basic properties of a solution, resulting in a close to neutral pH. Examples of common acids and bases include hydrochloric acid, sulfuric acid, sodium hydroxide, and potassium hydroxide.
Common Properties of Bases
Bases are a class of substances that have distinct properties and characteristics. Understanding these properties can help us identify and differentiate bases from other substances. Some common properties of bases include:
- Taste: Bases taste bitter or soapy.
- Touch: Bases have a slippery or soapy feel.
- Color change: Bases can cause certain dyes to change color. For example, red cabbage juice turns dark green or blue in the presence of a base.
- Corrosive: Bases can be corrosive and cause damage to living tissue or certain materials like metals.
- React with acids: Bases react with acids to form salt and water in a chemical reaction called neutralization.
- Electrical conductivity: Bases are generally electrolytes and can conduct electricity when dissolved in water.
These properties are useful in identifying bases and understanding their behavior in various chemical reactions. It is important to note that not all bases exhibit these properties to the same extent, and there may be variations depending on the specific base being studied.
How Do Bases React with Acids?
Bases and acids are two types of substances that have contrasting properties and react with each other in various ways. When bases react with acids, a chemical reaction known as neutralization occurs. This reaction involves the transfer of protons (H+ ions) from the acid to the base, resulting in the formation of water and a salt.
During the neutralization process, the base accepts the H+ ions from the acid, which leads to the formation of water. This is because bases have the ability to accept protons due to their structure and properties. The reaction between a base and an acid can be represented by the general equation: base + acid → salt + water.
For example, if we consider the reaction between sodium hydroxide (a base) and hydrochloric acid, the equation would be: NaOH + HCl → NaCl + H2O. In this reaction, sodium hydroxide accepts the proton from hydrochloric acid, resulting in the formation of sodium chloride (salt) and water.
The strength of a base, as well as the concentration of both the base and the acid, influences the reaction rate and the amount of product formed. Strong bases, such as sodium hydroxide and potassium hydroxide, react more readily with acids compared to weak bases. Additionally, higher concentrations of both the base and the acid will lead to a faster reaction.
Importance of Understanding Acids and Bases
Understanding acids and bases is essential in various fields of science and everyday life. Acids and bases play a crucial role in chemical reactions, healthcare, environmental studies, and even household activities. They are fundamental concepts that help us comprehend and predict how substances interact with each other.
In Chemistry: Acids and bases are the foundation of chemical reactions. They have distinct properties and can neutralize each other. Understanding their characteristics, such as pH levels and strength, is crucial for determining reaction outcomes. This knowledge is vital in fields like chemical synthesis, pharmaceuticals, and material science, where acids and bases are routinely used.
In Healthcare: Acids and bases are also significant in medical applications. For example, in pharmacology, understanding the acidity or basicity of a drug is essential for its effectiveness and stability. In dentistry, knowledge of acids and bases helps dentists diagnose and treat dental problems, such as tooth decay and enamel erosion, caused by acids produced by bacteria.
In Environmental Studies: Understanding the impact of acids and bases on the environment is crucial for monitoring and managing pollution. Acidic rain, for instance, can harm ecosystems and damage buildings and infrastructure. Knowledge of acids and bases helps scientists and environmentalists identify the sources of acidity and develop strategies to reduce pollution and its harmful effects.
In Everyday Life: Acids and bases are present in numerous household products, such as cleaning agents, personal care items, and even food and beverages. Understanding their properties and reactions can help us select the right product for a specific task and handle them safely. For example, knowledge of acidic and basic solutions can help us prevent accidents and injury by using appropriate protective measures when handling or storing certain substances.
In conclusion, understanding acids and bases is vital in various scientific disciplines and practical applications. Whether in chemistry, healthcare, environmental studies, or everyday life, this knowledge allows us to comprehend how substances interact, make informed decisions, and ensure the safety and well-being of ourselves and the environment.
Answer Key to the Acids and Bases Worksheet
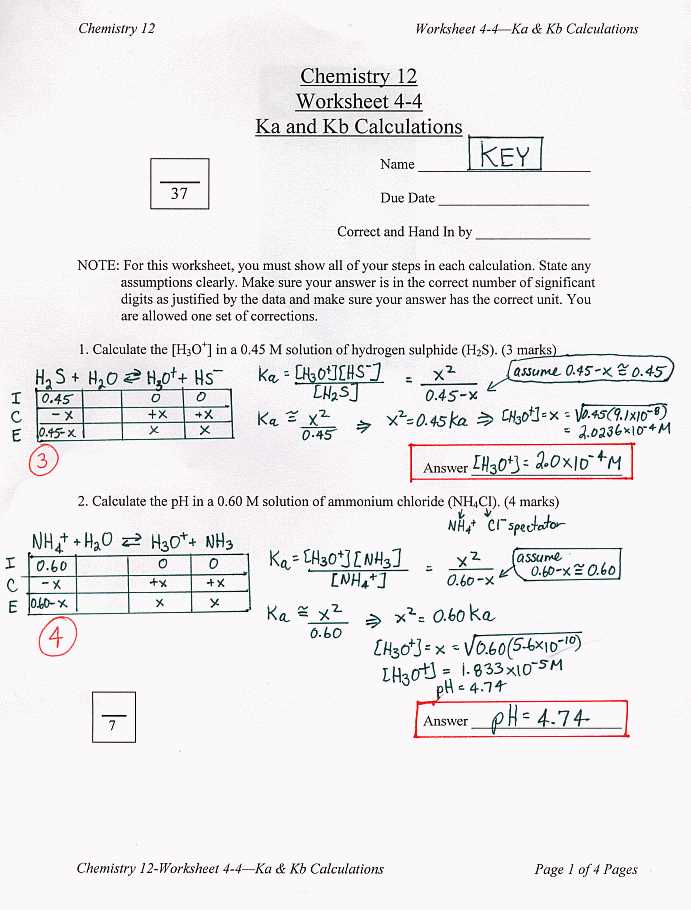
In the study of chemistry, acids and bases play a crucial role. Understanding the properties and behavior of these substances is fundamental to many fields, including biochemistry, medicine, and environmental science. The “Acids and Bases Worksheet” provides students with an opportunity to test and apply their knowledge on this topic. Here, we present the answer key to help students evaluate their understanding and improve their learning.
Section 1: Acid-Base Definitions
1. Strong acids are defined as substances that completely dissociate in water, producing a high concentration of hydrogen ions (H+). Examples of strong acids include hydrochloric acid (HCl) and sulfuric acid (H2SO4).
2. Weak acids, on the other hand, only partially dissociate in water, leading to a lower concentration of hydrogen ions. Acetic acid (CH3COOH) and carbonic acid (H2CO3) are examples of weak acids.
3. Bases are substances that accept protons or donate electron pairs. Strong bases completely dissociate in water, resulting in a high concentration of hydroxide ions (OH-). Sodium hydroxide (NaOH) and potassium hydroxide (KOH) are examples of strong bases.
4. Weak bases, such as ammonia (NH3) and aluminum hydroxide (Al(OH)3), only partially dissociate in water, reducing the concentration of hydroxide ions.
Section 2: Acid-Base Reactions
1. In an acid-base reaction, the acid donates a proton (H+) to the base, forming a water molecule. This is known as a neutralization reaction, and the products are a salt and water.
2. For example, when hydrochloric acid (HCl) reacts with sodium hydroxide (NaOH), the hydrogen ion (H+) from the acid combines with the hydroxide ion (OH-) from the base to form water (H2O). The remaining sodium ion (Na+) and chloride ion (Cl-) combine to form sodium chloride (NaCl).
3. Acid-base reactions can also involve weak acids or bases. These reactions are not as complete as those with strong acids or bases, and the products may still contain some partially dissociated ions.
Section 3: pH Scale
1. The pH scale is used to measure the acidity or basicity of a substance. It ranges from 0 to 14, with 0 being the most acidic, 7 being neutral, and 14 being the most basic.
2. Substances with a pH less than 7 are acidic, while those with a pH greater than 7 are basic. A pH of 7 indicates neutrality.
3. The pH of a substance is determined by the concentration of hydrogen ions. Higher concentrations of hydrogen ions result in a lower pH, indicating greater acidity. Conversely, lower concentrations of hydrogen ions lead to a higher pH, indicating greater basicity.
Section 4: Indicators
1. Indicators are substances that change color depending on the acidity or basicity of a solution. One example is litmus paper, which turns red in the presence of an acid and blue in the presence of a base.
2. Other indicators include phenolphthalein, which is colorless in acidic solutions and pink in basic solutions, and bromothymol blue, which is yellow in acidic solutions and blue in basic solutions.
3. These indicators are useful tools in determining the pH of a solution, especially when precise measurements are not necessary. However, they may not provide an accurate measurement of pH values.
Section 5: Buffer Systems
1. Buffer systems are mixtures of weak acids or bases and their conjugate salts that help maintain a relatively constant pH in a solution.
2. When an acid or base is added to a buffer system, the buffer components react to minimize the changes in pH. The weak acid or base in the buffer resists the addition of protons or hydroxide ions, maintaining the pH within a specific range.
3. Buffers are essential in biological systems, such as blood, where even slight changes in pH can have detrimental effects on cellular functions.
By using this answer key and reviewing the explanations provided, students can assess their understanding of acids and bases, ensuring they have a solid foundation in this important area of chemistry.