
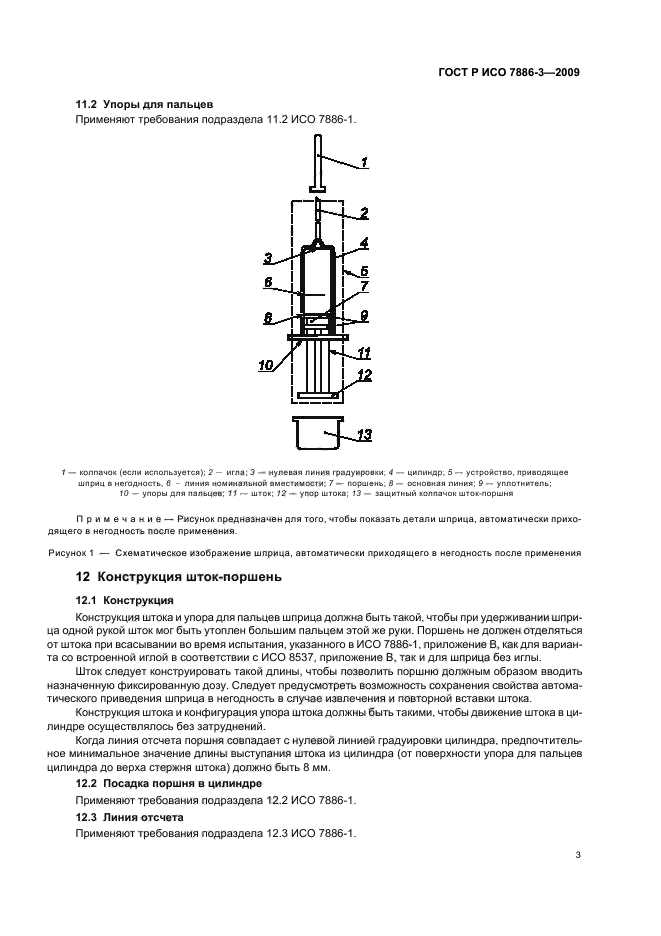
Hypodermic syringes are a crucial medical device used for administering injections, vaccinations, and medications. To ensure their safety and quality, international standards like ISO 7886-1 provide guidelines for testing these syringes. This article explores the importance of ISO 7886-1 testing and its impact on the healthcare industry.
What is ISO 7886-1 Testing?
ISO 7886-1 is a standard developed by the International Organization for Standardization (ISO) that specifically addresses the requirements for hypodermic syringes. It focuses on the testing and evaluation of syringes to ensure their safety, accuracy, and reliability in delivering medications to patients.
The testing conducted according to ISO 7886-1 involves various parameters such as leakage, needle pull-out force, measurement accuracy, and functional performance. These tests evaluate the syringe’s ability to deliver the intended dose, prevent leakage, and maintain needle stability during injection.
The Importance of ISO 7886-1 Testing
ISO 7886-1 testing is crucial for several reasons:
- Patient Safety: By adhering to the standards outlined in ISO 7886-1, manufacturers ensure that the syringes they produce are safe and reliable for patients. The testing helps identify any potential risks or defects that may compromise the patient’s wellbeing.
- Accuracy of Medication Delivery: ISO 7886-1 testing verifies the accuracy of the syringe in delivering the intended dose of medication. This is especially important for critical injections where dosage precision is vital for effective treatment.
- Prevention of Needlestick Injuries: The testing includes evaluating the needle’s stability and pull-out force to minimize the risk of needle detachment during injection. This helps prevent needlestick injuries and ensures healthcare workers’ safety.
- Standardization: ISO 7886-1 provides a standardized framework for testing hypodermic syringes. This consistency allows healthcare professionals and regulatory bodies to compare and evaluate syringes easily, making informed decisions about their use.
- Quality Control: By conducting ISO 7886-1 testing, manufacturers can ensure the quality of their syringes and maintain consistency in production. This helps build trust in the brand and contributes to overall healthcare system integrity.
The Impact on the Healthcare Industry

The implementation of ISO 7886-1 testing has had a significant impact on the healthcare industry:
- Improved Patient Care: With syringes meeting the ISO 7886-1 standards, patients can receive accurate dosages of medications, leading to improved treatment outcomes and better overall care.
- Reduced Risk of Contamination: ISO 7886-1 testing ensures that syringes have proper sealing mechanisms, minimizing the risk of contamination during storage and transportation. This plays a vital role in reducing infections and maintaining patient safety.
- Enhanced Healthcare Provider Safety: By evaluating needle stability and pull-out force, ISO 7886-1 testing helps prevent needlestick injuries among healthcare providers. This promotes a safer working environment and decreases the associated healthcare costs.
- Global Compatibility: The adoption of ISO 7886-1 as an international standard enables compatibility and interchangeability of syringes across different healthcare settings worldwide. This facilitates global healthcare delivery and ensures consistent quality.
Overall, ISO 7886-1 testing plays a crucial role in ensuring the safety, accuracy, and reliability of hypodermic syringes. By adhering to these standards, manufacturers and healthcare professionals can provide better patient care and contribute to the overall improvement of the healthcare industry.
What is ISO 7886-1?

ISO 7886-1 is an international standard that specifies the requirements for single-use hypodermic syringes with a needle mount for medical purposes. The standard provides guidelines for the design, construction, and performance of these syringes, ensuring their safety and efficiency in healthcare settings.
The ISO 7886-1 standard covers various aspects of the syringe, including its dimensions, materials, and performance characteristics. It defines the requirements for the syringe barrel, plunger, needle mount, and other components, ensuring compatibility and functionality. The standard also includes requirements for the sterility of the syringe, packaging, and labeling.
The ISO 7886-1 standard is essential in ensuring the safety and effectiveness of hypodermic syringes used in medical procedures. By providing clear guidelines and criteria, the standard helps manufacturers produce high-quality syringes that meet the needs of healthcare professionals and patients. It also aims to minimize the risk of needlestick injuries and infections associated with the use of syringes.
Compliance with ISO 7886-1 is fundamental for syringe manufacturers to demonstrate the quality and safety of their products. Healthcare facilities and professionals rely on these standards to select syringes that meet the necessary requirements for their specific applications. Adhering to the standard also ensures that syringes are manufactured in a consistent and reliable manner, promoting trust and confidence in the medical device industry.