
Understanding the Lewis structure of molecules is critical for understanding their chemical properties and behavior. The Lewis structure provides a visual representation of how atoms are connected within a molecule and the distribution of electrons. By understanding Lewis structures, chemists can predict the reactivity and stability of molecules.
A Lewis structure is made up of symbols representing atoms, connected by lines representing chemical bonds. Electrons are depicted as dots or lines around the atoms to show their distribution. The arrangement of atoms and electrons in a Lewis structure helps determine the shape, polarity, and overall stability of a molecule.
The Lewis structure answer key serves as a guide to help determine the correct arrangement of atoms and electrons. It provides a set of rules and guidelines for constructing Lewis structures accurately. By following the answer key, chemists can avoid common mistakes and ensure the validity of their structures.
Overall, the Lewis structure answer key is an essential tool for understanding and predicting the behavior of molecules. It helps chemists create accurate representations of molecular structures, allowing for a better understanding of their properties and reactivity. By mastering the use of Lewis structures, chemists can make informed decisions in diverse areas such as drug development, materials science, and environmental chemistry.
Lewis Structure Answer Key: Understanding the Basics

In chemistry, Lewis structures are visual representations of the electron distribution in a molecule. These structures are named after Gilbert N. Lewis, who first introduced them in 1916. Lewis structures are an essential tool for understanding the bonding and structure of molecules.
The Lewis structure answer key provides a guide for drawing Lewis structures correctly. It helps chemists identify the correct number of valence electrons for each atom and determine the bonding and non-bonding pairs of electrons. The key is especially useful for beginners who are learning how to draw Lewis structures and need assistance in identifying the correct placement of electrons.
The key consists of a step-by-step process:
- Determine the number of valence electrons for each atom in the molecule. This can be done by referring to the periodic table.
- Determine the central atom. The central atom is usually the least electronegative atom in the molecule, except in cases where hydrogen or halogens are present. Carbon is typically the central atom in organic molecules.
- Connect all atoms with single bonds to the central atom.
- Distribute the remaining electrons to satisfy the octet rule. This rule states that atoms tend to gain, lose, or share electrons in order to achieve a stable configuration with eight valence electrons.
- If the central atom does not have an octet, form multiple bonds by converting lone pairs of electrons into bonding pairs.
- Check the formal charges of each atom. Formal charge is a measure of the electron distribution in a molecule and can help identify the most stable Lewis structure.
- Verify that the Lewis structure obeys the octet rule and does not violate any other chemical principles.
Overall, the Lewis structure answer key serves as a valuable tool for understanding the basics of drawing Lewis structures. It provides a systematic approach to evenly distribute electrons and ensure the stability of the molecule. By following the steps outlined in the key, chemists can accurately represent the electron distribution and predict the chemical behavior of molecules.
What is a Lewis Structure?
A Lewis structure, also known as a Lewis dot diagram, Lewis dot structure, or electron dot structure, is a visual representation used to show the arrangement of electrons in a molecule or ion. It was introduced by the American chemist Gilbert N. Lewis in 1916 as a simple way to understand the bonding and electron distribution in chemical compounds.
The Lewis structure consists of chemical symbols for the elements and dots or lines to represent the valence electrons. Valence electrons are the electrons in the outermost energy level of an atom that are involved in chemical bonding. The dots or lines are placed around the respective chemical symbols to indicate the number of valence electrons in an atom.
In a Lewis structure, each dot represents one valence electron, while a line represents a pair of electrons. The goal of creating a Lewis structure is to arrange the electrons in a way that satisfies the octet rule, which states that atoms tend to gain, lose, or share electrons in order to achieve a stable configuration with 8 valence electrons.
By examining the Lewis structure of a molecule, one can determine the type of bonding present, whether it is covalent or ionic, as well as the overall shape and polarity of the molecule. Lewis structures are widely used in chemistry to understand the behavior of molecules and predict their chemical properties.
Importance of Lewis Structures in Chemistry
Lewis structures, also known as Lewis dot structures or electron dot structures, are diagrams used to represent the arrangement of atoms and valence electrons in a molecule or ion. They were introduced by Gilbert N. Lewis in 1916 and have since become a fundamental tool in the field of chemistry. Lewis structures provide a visual representation of the bonding and non-bonding electrons in a molecule, allowing chemists to predict the molecular geometry, bond angles, and overall chemical behavior.
One of the key benefits of Lewis structures is their ability to help predict the reactivity and stability of molecules. By examining the arrangement of electrons and the connectivity between atoms, chemists can determine which molecules are more likely to undergo chemical reactions and which ones are more stable. For example, molecules with complete octets or filled valence shells are generally more stable and less reactive compared to those with incomplete octets or unpaired electrons. By analyzing Lewis structures, chemists can predict the likelihood of chemical reactions and design new compounds with desired properties.
Lewis structures are also essential in understanding and explaining the concept of chemical bonding. They allow chemists to identify different types of chemical bonds, such as covalent bonds, in which electrons are shared between atoms, and ionic bonds, in which electrons are transferred from one atom to another. By drawing Lewis structures, chemists can visualize the distribution of electrons and determine the type of bond formed between atoms. This knowledge is crucial in understanding the physical and chemical properties of substances, as different types of bonds result in varying degrees of polarity and strength.
In addition, Lewis structures play a crucial role in the study of organic chemistry, where they are used to represent the structures of complex carbon-based molecules. By drawing Lewis structures for organic compounds, chemists can analyze the connectivity of carbon atoms, predict the presence of functional groups, and understand the overall structure and reactivity of organic molecules. This information is essential for the synthesis of new organic compounds and the development of drugs, materials, and other important chemical products.
In conclusion, Lewis structures are an indispensable tool in chemistry, enabling chemists to understand the structure, bonding, and reactivity of molecules. They provide a visual representation of electron distribution, helping to predict molecular geometry, bond angles, and overall chemical behavior. Whether in the analysis of inorganic compounds, organic molecules, or complex ions, Lewis structures are an essential part of the chemist’s toolkit.
How to Draw Lewis Structures
Lewis structures are diagrams that show the bonding between atoms and the lone pairs of electrons that may exist in a molecule or ion. These structures help chemists understand the arrangement of electrons within a compound and predict its properties. Drawing Lewis structures can be a useful tool in studying and interpreting different chemical reactions.
To draw a Lewis structure, follow these steps:
- Step 1: Count the total number of valence electrons in the molecule or ion. This can be done by adding up the number of valence electrons from each atom.
- Step 2: Determine the central atom, which is usually the least electronegative atom. This atom will be the central hub for bonding with other atoms.
- Step 3: Connect the atoms using single bonds. Each bond represents two electrons. Place the bonded atoms around the central atom.
- Step 4: Place the remaining electrons around the atoms to satisfy the octet rule. Each atom (except hydrogen) should have eight electrons, either in the form of lone pairs or shared bonds.
- Step 5: If there are remaining electrons after satisfying the octet rule, place them as lone pairs on the central atom.
- Step 6: If the central atom does not have an octet after following steps 4 and 5, form double or triple bonds to satisfy the octet rule.
It is important to note that there are some exceptions to the octet rule. For example, atoms such as boron and beryllium can have fewer than eight electrons around them.
Overall, drawing Lewis structures is a valuable skill in chemistry. It allows chemists to visualize the arrangement of electrons in a compound and predict its behavior in different reactions. By following the steps outlined above, one can effectively draw Lewis structures for various molecules and ions.
Key Guidelines for Drawing Lewis Structures
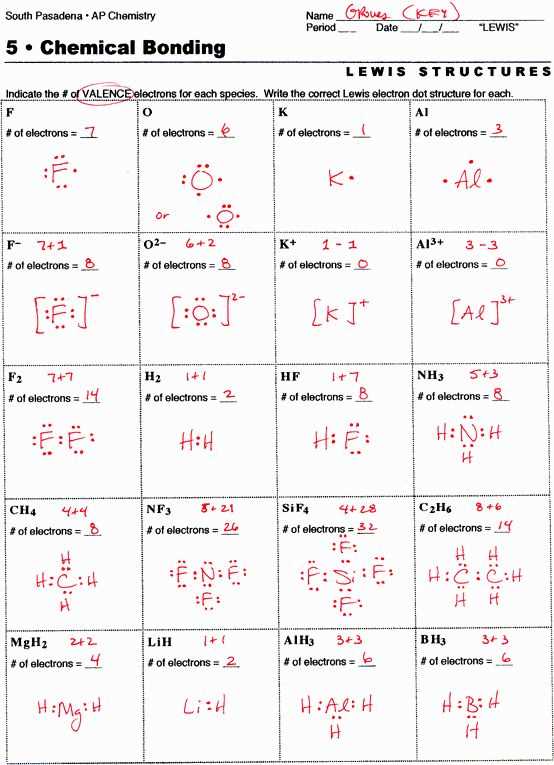
When drawing Lewis structures, it is essential to follow some key guidelines to accurately represent the structure and bonding of molecules. These guidelines help in determining the correct arrangement of atoms and the sharing of electrons in a molecule.
1. Count the Total Number of Valence Electrons:
The first step in drawing a Lewis structure is to count the total number of valence electrons of all atoms involved in the molecule. This can be done by referring to the periodic table and considering the group number of each atom. For example, in a water molecule (H2O), oxygen belongs to group 16, so it carries 6 valence electrons, while hydrogen belongs to group 1 and carries 1 valence electron each. The total number of valence electrons in water is then calculated as 6 (oxygen) + 2 (hydrogen) = 8 valence electrons.
2. Determine the Central Atom:
The next step is to determine the central atom in the molecule. The central atom is usually the one that has the lowest electronegativity and the highest valence. In water, oxygen is the central atom as it can accommodate more than one bond.
3. Connect the Atoms with Single Bonds:
After determining the central atom, connect the other atoms to it using single bonds. In water, two hydrogen atoms are connected to the central oxygen atom by single bonds. These bonds are represented as lines between the atoms.
4. Distribute the Remaining Electrons:
Once the single bonds are drawn, distribute the remaining valence electrons around the atoms in pairs to satisfy the octet rule, except for hydrogen, which only needs two electrons to fill its valence shell. The octet rule states that atoms tend to gain, lose, or share electrons to achieve a stable configuration with 8 electrons in their valence shell. For example, in water, oxygen has already used 2 valence electrons for the bonds with hydrogen, so 6 electrons remain. Distribute them as two lone pairs around the oxygen atom.
By closely following these key guidelines, one can accurately draw a Lewis structure that correctly represents the molecular structure and bonding. It is important to note that exceptions to the octet rule may occur for molecules with an odd number of electrons or elements from groups 13-18.
Lewis Structure Examples
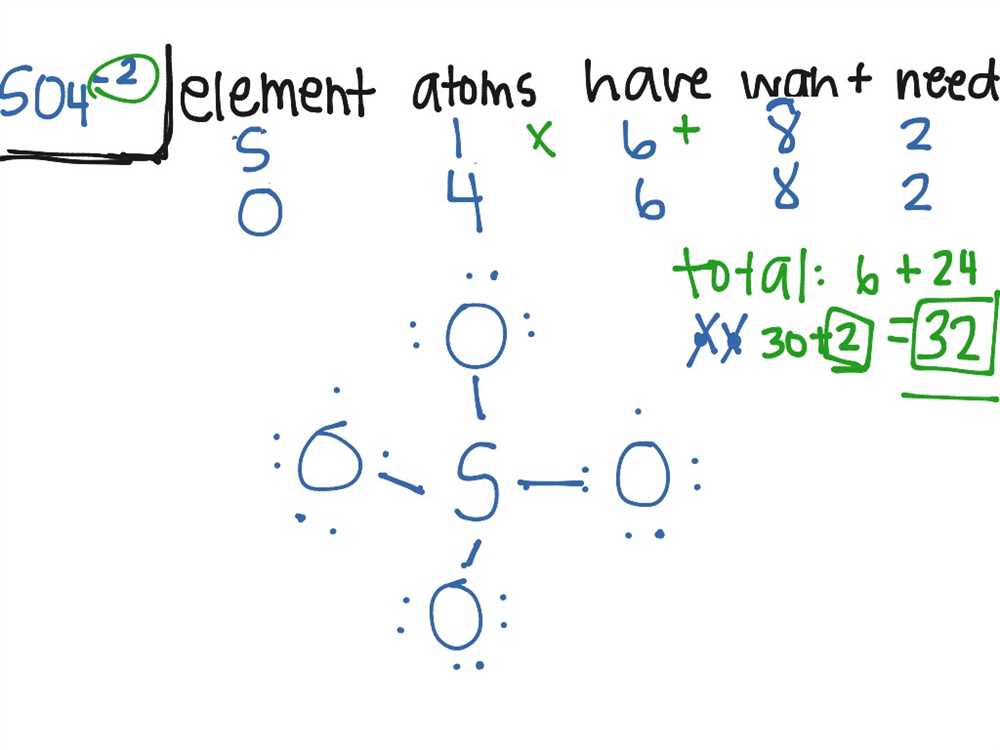
A Lewis structure is a diagram that represents the bonding between atoms in a molecule or ion along with any lone pairs of electrons present. It provides a visual representation of the electron distribution in a molecule, which is important for understanding its chemical properties and reactivity. Here are a few examples of Lewis structures to illustrate how they are constructed.
Example 1: Water (H2O)
 H
H
O
:
O
:
O
:
H
H
H
H
O
:
O
:
O
:
H
H
Example 2: Carbon Dioxide (CO2)
Carbon dioxide consists of one carbon atom bonded to two oxygen atoms. Carbon has four valence electrons, while each oxygen atom has six valence electrons. The total number of valence electrons in CO2 is 16. The Lewis structure shows carbon as the central atom bonded to two oxygen atoms using shared electron pairs. The oxygen atoms each have two lone pairs of electrons.
- Carbon (C): 4 valence electrons
- Oxygen (O): 6 valence electrons
| O | O | ||||
| C | = | C | = | C | = |
| O | O |
These examples demonstrate how Lewis structures can be used to visualize the electron distribution in molecules and ions. By understanding the arrangement of electrons, chemists can analyze and predict chemical reactions and properties.