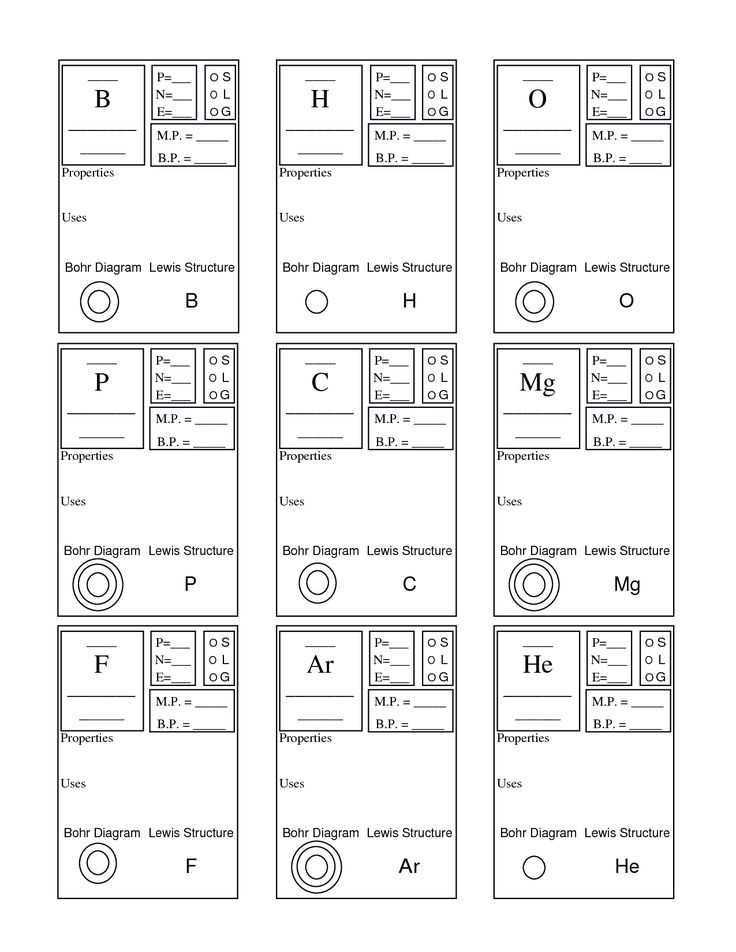
Lewis structures, also known as electron dot structures, are diagrams that show the bonding between atoms in a molecule and the lone pairs of electrons that may exist. Understanding how to read and interpret Lewis structures is crucial for predicting molecular geometries and determining the polarity of molecules.
In this Lewis structure worksheet 1 answer key, we will explore the basics of drawing Lewis structures and understanding the rules and principles that govern them. By learning how to correctly place electrons and determine the formal charges of atoms, you will be able to construct accurate Lewis structures for various molecules.
This answer key will provide step-by-step explanations and solutions for the Lewis structure worksheet 1, helping you develop a strong foundation in molecular structure and furthering your understanding of chemical compounds and their properties.
Lewis Structure Worksheet 1 Answer Key
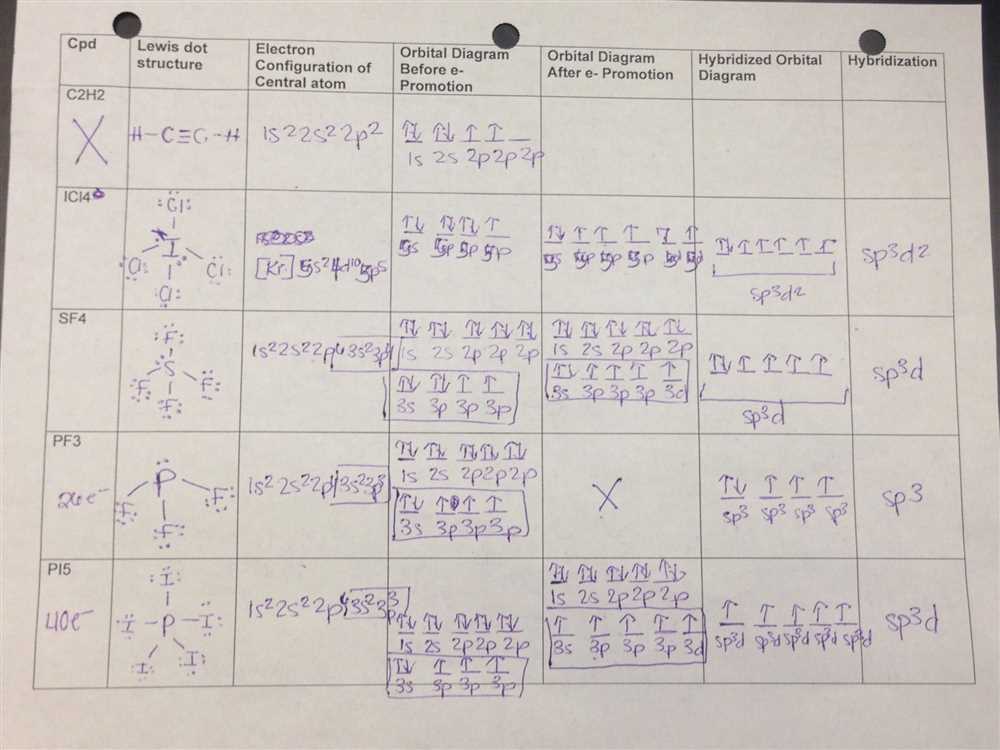
Below is the answer key for Lewis Structure Worksheet 1, which is designed to help students practice drawing Lewis structures for covalent compounds and polyatomic ions. These Lewis structures show the arrangement of atoms and the bonding electrons in a molecule or ion.
1. XeF4 – This compound is formed by the central xenon atom bonded to four fluorine atoms. The Lewis structure shows that xenon has two lone pairs and forms four single bonds with the fluorine atoms.
2. NH3 – The Lewis structure for ammonia shows that the central nitrogen atom is bonded to three hydrogen atoms and has one lone pair. The lone pair on nitrogen gives ammonia its characteristic shape and chemical properties.
3. CO2 – The Lewis structure for carbon dioxide shows that the central carbon atom forms a double bond with each oxygen atom. Carbon dioxide is a linear molecule due to its symmetric arrangement of atoms and double bonds.
4. H2>O – The Lewis structure for water shows that the central oxygen atom is bonded to two hydrogen atoms and has two lone pairs. These lone pairs give water its bent shape and are responsible for its unique properties such as polarity and hydrogen bonding.
5. BrF5 – This compound is formed by the central bromine atom bonded to five fluorine atoms. The Lewis structure shows that bromine has three lone pairs and forms five single bonds with the fluorine atoms.
Overall, Lewis structures are a useful tool for visualizing the molecular structure and bonding in covalent compounds and polyatomic ions. They provide a way for students to understand and predict the behavior of molecules based on their structural arrangements and electron distribution.
What is a Lewis Structure?
A Lewis structure, also known as a Lewis dot diagram, is a visual representation of the valence electrons in an atom or a molecule. It was introduced by Gilbert N. Lewis in 1916 as a way to depict the bonding between atoms in a simple and easy-to-understand manner. The Lewis structure shows the arrangement of electrons, both shared and unshared, around the atoms in a molecule.
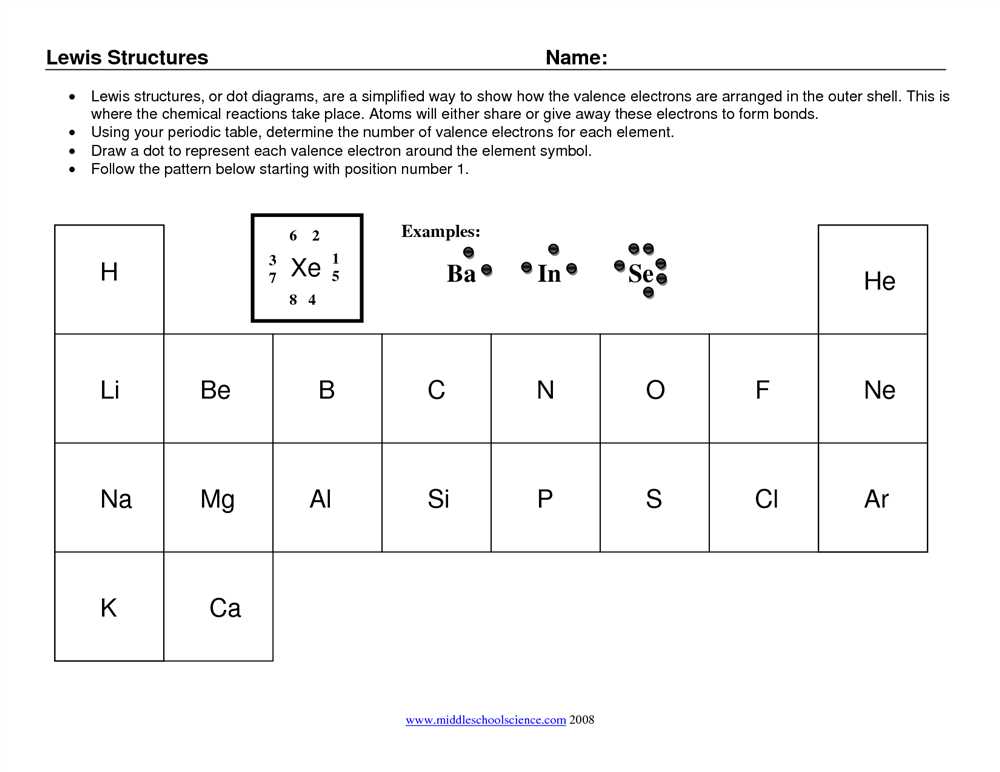
Each element in the periodic table has a specific number of valence electrons, which are the electrons in the outermost energy level of an atom. In a Lewis structure, these valence electrons are represented as dots placed around the atomic symbol. The number of dots corresponds to the number of valence electrons. For example, carbon has four valence electrons, so its Lewis structure would have four dots around the atomic symbol.
The Lewis structure also includes lines to represent covalent bonds between atoms. A covalent bond is formed when two atoms share a pair of electrons. In a Lewis structure, a line is used to represent a pair of shared electrons. For example, in a molecule of water (H2O), the oxygen atom is bonded to two hydrogen atoms. The Lewis structure of water would show two lines between the oxygen atom and each hydrogen atom, indicating the shared electron pairs.
By studying Lewis structures, chemists can understand the bonding and structure of molecules, which is essential in predicting their properties and reactivity. Lewis structures are a fundamental tool in chemistry and serve as the starting point for more advanced topics such as molecular orbital theory and resonance.
Key Concepts in Lewis Structure
The concept of Lewis structure is essential in understanding the arrangement of atoms and electrons within a molecule. Lewis structures depict the bonding and non-bonding electron pairs present in a compound or molecule. By following specific guidelines and rules, we can represent the distribution of electrons in a clear and organized manner.
1. Octet Rule: The octet rule states that atoms tend to gain, lose, or share electrons in order to acquire a stable electron configuration with eight valence electrons. This rule helps determine the number and types of bonds formed in a Lewis structure.
2. Valence Electrons: Valence electrons are the outermost electrons of an atom. These electrons are involved in bonding and are represented by dots in a Lewis structure. The number of valence electrons of an atom can be determined by its position on the periodic table.
3. Lone Pairs: Lone pairs are non-bonding electrons that are not involved in any chemical bonding. These electrons are represented by pairs of dots in a Lewis structure. Lone pairs have an influence on the overall shape and polarity of a molecule.
4. Bonding Pairs: Bonding pairs are electrons that are shared between two atoms. These electrons form covalent bonds and are represented by lines or dashes in a Lewis structure. Bonding pairs help determine the connectivity and arrangement of atoms within a molecule.
5. Formal Charge: Formal charge is a concept used to evaluate the distribution of electrons in a Lewis structure. It is calculated by subtracting the number of lone pairs and half the number of bonding electrons from the total valence electrons of an atom. Formal charges help determine the most stable resonance structure.
In conclusion, understanding key concepts in Lewis structure is crucial for accurately representing the electron distribution and bonding in a molecule. By utilizing these concepts, we can visually depict the arrangement of atoms and electrons, and make predictions about the properties and behavior of compounds.
How to Draw Lewis Structures
A Lewis structure is a diagram that shows the arrangement of atoms and the bonding between them in a molecule. It is a useful tool in chemistry for understanding molecular structure and predicting chemical behavior. Drawing Lewis structures requires an understanding of valence electrons and the rules that govern their distribution.
To draw a Lewis structure, follow these steps:
- Count the total number of valence electrons for all the atoms in the molecule.
- Determine the central atom in the molecule. This is usually the least electronegative atom and the one that forms the most bonds.
- Connect the atoms in the molecule with single bonds. The total number of electrons used in the bonds must be subtracted from the total number of valence electrons.
- Place the remaining electrons on the outer atoms to satisfy the octet rule. Each atom should have eight electrons around it, except for hydrogen, which only needs two.
- If there are still remaining electrons, place them on the central atom as lone pairs to satisfy the octet rule.
- Check if all atoms have satisfied the octet rule. If not, convert lone pairs on outer atoms into double or triple bonds to satisfy the octet rule.
It is important to note that Lewis structures provide a simplified representation of molecules and do not account for the three-dimensional shape of the molecule. However, they serve as a useful starting point for understanding molecular structure and predicting chemical properties.
Practice Problems
Here are some practice problems to help you better understand Lewis structures:
Problem 1:
Draw the Lewis structure for carbon dioxide (CO2).
Solution:
- Start by counting the number of valence electrons for each atom. Carbon has 4 valence electrons, while oxygen has 6 valence electrons.
- Place the least electronegative atom (carbon) in the center.
- Connect the oxygen atoms to the carbon atom using single bonds.
- Distribute the remaining valence electrons around the atoms to satisfy the octet rule.
Problem 2:
Draw the Lewis structure for methane (CH4).
Solution:
- Start by counting the number of valence electrons for each atom. Carbon has 4 valence electrons, while hydrogen has 1 valence electron.
- Place the carbon atom in the center and connect hydrogen atoms to it using single bonds.
- Distribute the remaining valence electrons around the atoms to satisfy the octet rule.
Problem 3:
Draw the Lewis structure for water (H2O).
Solution:
- Start by counting the number of valence electrons for each atom. Hydrogen has 1 valence electron, while oxygen has 6 valence electrons.
- Place the oxygen atom in the center and connect hydrogen atoms to it using single bonds.
- Distribute the remaining valence electrons around the atoms to satisfy the octet rule.
Continue practicing with more molecules to improve your skills in drawing Lewis structures.
Tips for Drawing Lewis Structures
When it comes to drawing Lewis structures, there are a few key tips to keep in mind. These structures are representations of the way atoms are connected in a molecule and provide important information about the molecule’s shape and chemical properties. Here are some helpful tips to guide you through the process:
- Count the total number of valence electrons: To begin drawing a Lewis structure, you need to determine the total number of valence electrons in the molecule. This can be done by adding the number of valence electrons for each atom. For example, oxygen has six valence electrons, and carbon has four.
- Identify the central atom: In most cases, the least electronegative atom becomes the central atom. This atom is usually the one that can form the most bonds. Hydrogen is never the central atom.
- Distribute the electrons: Distribute the valence electrons around the atoms, starting with the central atom. Each bond (single, double, or triple) requires two electrons. Place lone pairs on the outer atoms first, and then distribute any remaining electrons on the central atom.
- Minimize formal charges: Try to arrange the atoms and electrons in a way that minimizes formal charges. Formal charges are used to indicate the distribution of electrons in a molecule and should ideally be as close to zero as possible.
- Check for octet rule violations: After distributing the electrons, check if any atoms have more or less than an octet of electrons. Hydrogen is an exception and can have a duet. If there are any violations, rearrange the bonding and lone pairs to achieve an octet or duet for each atom.
- Assign formal charges: Once the Lewis structure is complete, assign formal charges to each atom by comparing the number of valence electrons an atom has in the structure to its original number of valence electrons. Formal charges can help identify the most stable structure.
- Consider resonance structures: In some cases, a molecule can have multiple valid Lewis structures. These structures, known as resonance structures, differ only in the placement of electrons. Consider all possible resonance structures to fully represent the molecule.
By following these tips, you’ll be able to draw accurate and informative Lewis structures for various molecules, helping you better understand their properties and behaviors.
Common Mistakes in Lewis Structures
Understanding Lewis structures is crucial for studying and predicting the properties of molecules. However, there are several common mistakes that students often make when drawing Lewis structures. These mistakes can lead to incorrect representations of the molecular structure and can result in incorrect predictions of the molecule’s properties.
1. Failure to obey the octet rule

One of the most common mistakes is not properly following the octet rule. According to the octet rule, atoms tend to gain, lose, or share electrons in order to achieve a complete outer shell of eight electrons. When drawing Lewis structures, it is important to ensure that all atoms have a complete octet, except for hydrogen, which only needs two electrons. Ignoring the octet rule can lead to incorrect bonding and improper representation of the molecule.
2. Incorrect counting of valence electrons
Another common mistake is miscalculating the number of valence electrons in a molecule. Valence electrons are the electrons in the outermost energy level of an atom and are crucial for determining the bonding and structure of a molecule. It is important to correctly count the valence electrons for each atom in the Lewis structure in order to accurately represent the molecule.
3. Failure to consider formal charges
Formal charges are important for determining the most stable Lewis structure of a molecule. Formal charge is the charge that an atom would have if all the bonding electrons were equally shared between the bonded atoms. It is important to consider and calculate the formal charges of each atom in a Lewis structure. Failure to do so can result in an incorrect representation of the molecule’s structure and stability.
4. Ignoring resonance structures
Resonance structures occur when there are multiple ways to distribute the electrons in a molecule. It is important to consider and draw all possible resonance structures for a molecule in order to accurately represent its structure. Ignoring or neglecting resonance structures can lead to an incomplete and incorrect representation of the molecule.
Overall, understanding and properly drawing Lewis structures is crucial for studying and predicting the properties of molecules. Avoiding these common mistakes can help ensure an accurate representation of the molecule’s structure and properties.