Lewis structures, also known as Lewis dot diagrams or electron dot diagrams, are simple drawings used in chemistry to represent the arrangement of electrons in a molecule or ion. They were developed by American chemist Gilbert N. Lewis in 1916 and are still an important tool in understanding chemical bonding and molecular structure.
The purpose of Lewis structures is to provide a visual representation of the valence electrons, which are the outermost electrons of an atom involved in bonding. By using dots or lines to represent electrons, Lewis structures allow chemists to easily determine the number of valence electrons in a molecule and predict the type of chemical bonding that will occur.
In this part 1 of the Chem Worksheet 9 4, we will cover the basics of Lewis structures, including how to determine the number of valence electrons for an atom, how to draw Lewis structures for simple molecules, and how to identify lone pairs and bonding pairs of electrons.
By understanding the concepts and techniques covered in this answer key, students will be well-equipped to tackle more complex Lewis structures in the future and gain a deeper understanding of chemical bonding and molecular structure.
Lewis Structures Part 1 Chem Worksheet 9 4 Answer Key
In chemistry, Lewis structures are used to represent the bonding between atoms in a molecule. They provide a visual representation of the electron distribution and help predict the shape, polarity, and reactivity of a molecule. Part 1 of the Chem Worksheet 9 4 focuses on understanding how to draw Lewis structures and determine the number of valence electrons in different elements.
The answer key for the Chem Worksheet 9 4 provides step-by-step explanations and solutions to the questions posed in the worksheet. It helps students verify their understanding and identify any mistakes in their calculations or drawings. The key also serves as a useful tool for instructors to assess students’ comprehension and provide feedback on their work.
To draw Lewis structures, students need to determine the total number of valence electrons in the molecule/ion, assign them to the appropriate atoms, and place any remaining electrons as lone pairs. They also need to consider the octet rule, which states that atoms tend to gain, lose, or share electrons to achieve a stable electron configuration with a full outer shell of electrons.
The Chem Worksheet 9 4 provides a series of exercises that challenge students to apply these concepts and draw correct Lewis structures for different molecules and ions. By using the answer key, students can compare their own work with the correct solutions and learn from any discrepancies.
Overall, the Lewis Structures Part 1 Chem Worksheet 9 4 Answer Key is an essential resource for students studying introductory chemistry. It helps them develop their skills in drawing Lewis structures and understanding the principles behind them, leading to a deeper understanding of chemical bonding and molecular properties.
What are Lewis structures and why are they important in chemistry?
Lewis structures are diagrams used in chemistry to represent the electron distribution in a molecule or ion. They are composed of symbols for atoms and lines to represent chemical bonds between them. Lewis structures provide a visual representation of how atoms are connected and the arrangement of valence (outermost) electrons in a molecule or ion.
One of the main reasons why Lewis structures are important in chemistry is that they help us predict the chemical behavior of molecules. By understanding the electron distribution and bonding patterns in a molecule, we can determine its reactivity, stability, and potential reactions with other substances. Lewis structures provide a way to analyze and compare different molecules, enabling us to make predictions about their physical and chemical properties.
Furthermore, Lewis structures are fundamental in understanding concepts such as molecular geometry and polarity. By examining the arrangement of atoms and lone pairs of electrons around a central atom, we can determine the shape of a molecule and whether it is polar or nonpolar. This information is crucial in understanding how molecules interact with each other and how they contribute to the properties of substances in the macroscopic world.
In summary, Lewis structures play a crucial role in chemistry by providing a visual representation of molecular structure and electron distribution. They allow us to predict the chemical behavior of molecules and understand concepts such as molecular geometry and polarity. By mastering the skill of drawing Lewis structures, chemists can gain valuable insights into the behavior and properties of substances, contributing to advancements in various fields, including medicine, materials science, and environmental studies.
How to draw Lewis structures for simple molecules?
The Lewis structure is a visual representation of the arrangement of atoms in a molecule and the bonding between them. It helps us understand the electron distribution and predict the molecular properties. Drawing Lewis structures for simple molecules involves following a set of guidelines:
- Step 1: Count the total number of valence electrons by adding up the valence electrons of all the atoms in the molecule.
- Step 2: Determine the central atom, which is usually the least electronegative element. Hydrogen is never the central atom.
- Step 3: Connect the peripheral atoms to the central atom using single bonds.
- Step 4: Distribute the remaining valence electrons around the atoms to satisfy the octet rule (except for hydrogen, which can have 2 electrons).
- Step 5: If there are any remaining valence electrons, place them on the central atom as lone pairs.
It’s important to remember that formal charges should be minimized in the Lewis structure. A formal charge is the charge an atom would have if all the bonding electrons were shared equally.
Once the Lewis structure is drawn, it can be used to determine the molecular geometry, bond angles, and polarity of the molecule. Lewis structures are a fundamental tool in understanding chemical bonding and predicting molecular behavior.
Understanding the Octet Rule and Its Role in Lewis Structures

The octet rule is a fundamental concept in chemistry that helps us understand the bonding patterns of atoms in molecules. It states that atoms tend to gain, lose, or share electrons in order to achieve a stable electron configuration with eight electrons in their outermost shell, also known as the valence shell.
The octet rule is based on the observation that noble gases have stable electron configurations with eight electrons in their valence shell, making them very stable and unreactive. Other elements in the periodic table strive to achieve a similar stable configuration by either gaining, losing, or sharing electrons with other atoms.
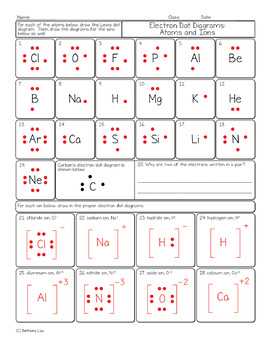
In Lewis structures, we represent atoms as symbols and use dots to represent valence electrons. The dots are placed around the symbol to indicate the number of valence electrons each atom has. By following the octet rule, we can determine how atoms bond and form molecules by sharing electrons.
For example, let’s consider the Lewis structure of methane (CH4). Carbon has four valence electrons, while hydrogen has one valence electron each. Carbon shares one electron with each of the four hydrogen atoms, forming four covalent bonds. This allows carbon to achieve a full octet (eight electrons) in its valence shell, while hydrogen achieves a duplet (two electrons) in its valence shell.
The octet rule provides a simple framework for understanding the stability and reactivity of atoms in molecules. It allows us to predict the arrangement of electrons in a molecule and explain why certain atoms form certain types of bonds. By applying the octet rule, we can draw Lewis structures that accurately represent the bonding patterns in a molecule, providing valuable insight into its chemical properties and behavior.
Steps to follow when drawing Lewis structures
When drawing Lewis structures, it is important to follow a systematic approach to ensure accuracy and clarity. Here are the steps to follow:
- Identify the central atom: Determine which atom will be in the center of the Lewis structure. This is usually the atom with the lowest electronegativity or the atom with the highest valence electron count.
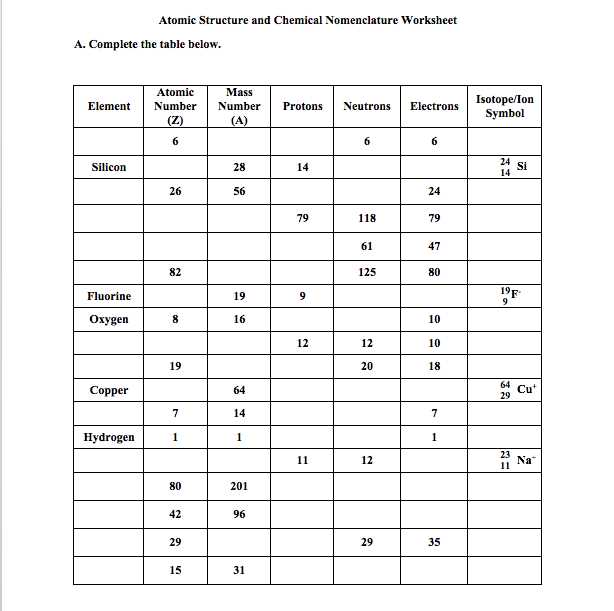
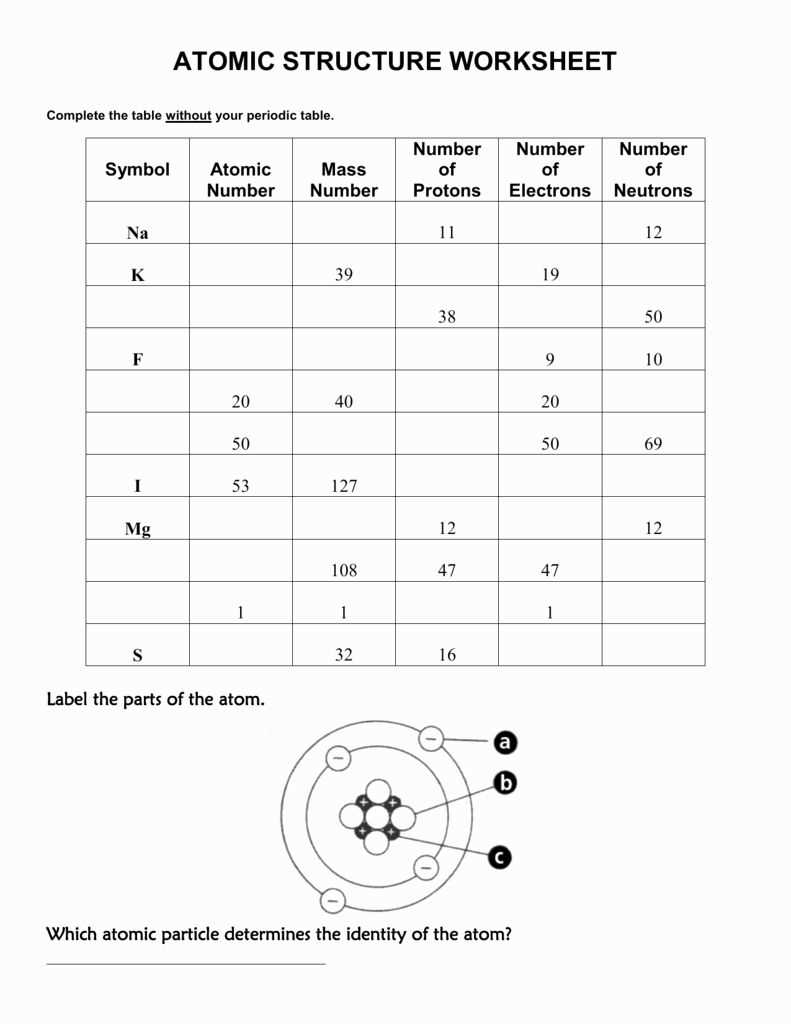
- Count valence electrons: Determine the total number of valence electrons for all the atoms in the molecule or ion. Remember to account for any charges present. Valence electrons are the electrons in the outermost energy level of an atom.
- Distribute electrons around the central atom: Start by placing pairs of electrons around the central atom, forming single bonds between the central atom and other atoms. Remember that each bond consists of two electrons.
- Complete octets: Fill the remaining valence electrons around the atoms, ensuring that each atom (except hydrogen) has a complete octet (8 electrons) or a duet (2 electrons). This can be achieved by forming double or triple bonds between atoms if necessary.
- Check formal charges: Calculate the formal charges for each atom in the Lewis structure to ensure that they are as close to zero as possible. Formal charges are used to determine the most stable arrangement of electrons in a molecule or ion.
- Review and refine: Evaluate the Lewis structure for any mistakes or inconsistencies. Make sure that all atoms have the correct number of valence electrons and that the overall charge of the molecule or ion is balanced.
Following these steps will help you accurately draw Lewis structures and understand the distribution of electrons in a molecule or ion.
Examples of Lewis Structures for Different Types of Molecules
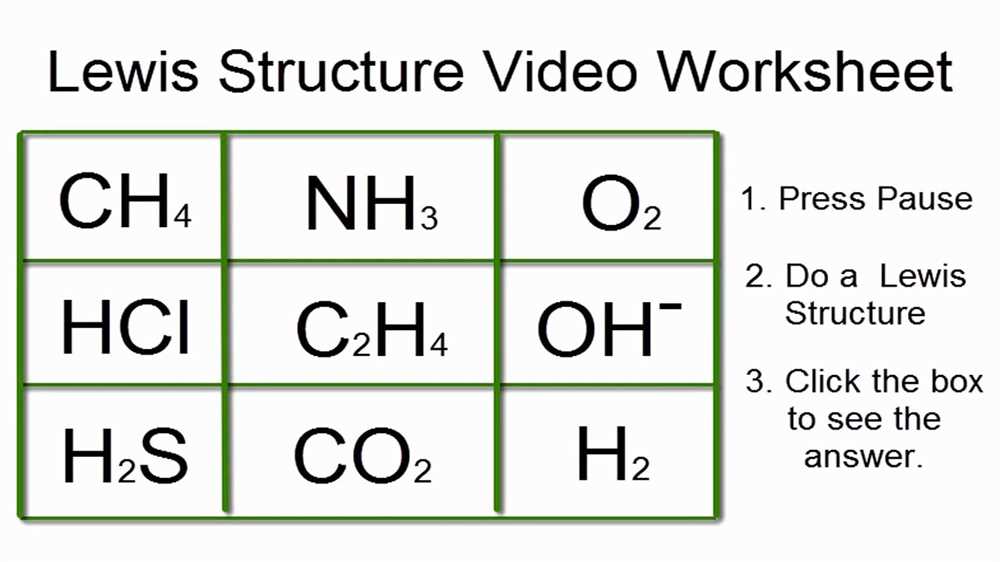
When it comes to representing the structure of molecules, Lewis structures provide a useful tool to understand the arrangement of atoms and their bonding in a molecule. Lewis structures use Lewis symbols, which consist of the element symbol surrounded by dots representing its valence electrons. Here are some examples of Lewis structures for different types of molecules:
Covalent Molecules
Covalent molecules are formed when atoms share electrons to achieve a stable electron configuration. In the Lewis structure for a covalent molecule, each shared pair of electrons is shown as a line connecting the two atoms. For example, in the Lewis structure for methane (CH4), the carbon atom is surrounded by four hydrogen atoms, and each hydrogen atom shares its single electron with the carbon atom.
Ionic Compounds
Ionic compounds are formed when one atom transfers electrons to another atom, resulting in the formation of ions with opposite charges that are attracted to each other. In the Lewis structure for an ionic compound, the ions are represented as individual Lewis structures, with the transfer of electrons shown as arrows. For example, in the Lewis structure for sodium chloride (NaCl), the sodium atom donates its valence electron to the chlorine atom, forming a positive sodium ion (Na+) and a negative chloride ion (Cl–).
Polar Molecules
Polar molecules have an uneven distribution of electron density, resulting in a partial positive charge on one end and a partial negative charge on the other end. In the Lewis structure for a polar molecule, the more electronegative atom is shown with an extra pair of electrons. For example, in the Lewis structure for water (H2O), the oxygen atom is more electronegative than the hydrogen atoms. Therefore, the oxygen atom is shown with two lone pairs of electrons, creating a partial negative charge on the oxygen atom and a partial positive charge on the hydrogen atoms.
- Overall, Lewis structures provide a visual representation of molecules and their bonding patterns. They help chemists predict the shape and properties of molecules, which is essential in understanding their behavior and reactivity.
- It is important to note that Lewis structures are a simplified representation of molecules and do not accurately depict the three-dimensional structure of molecules or their bond angles.
- However, Lewis structures are still valuable in understanding the basics of chemical bonding and providing a foundation for more advanced concepts in chemistry.
How to determine the formal charge in a Lewis structure
In chemistry, Lewis structures are diagrams that show the bonds and lone pairs of electrons in a molecule. They are an important tool for understanding the structure and properties of compounds. When drawing Lewis structures, it is crucial to determine the formal charge on each atom to ensure that the overall charge of the molecule is correctly represented.
To determine the formal charge in a Lewis structure, follow these steps:
- Count the number of valence electrons for each atom in the molecule. Valence electrons are the electrons in the outermost energy level of an atom.
- Assign each shared electron pair to one of the bonded atoms. This helps in determining the number of electrons each atom “owns”.
- Subtract one electron for each bond from the total number of valence electrons for that atom. This corresponds to the electrons it shares in the covalent bond.
- Add up the valence electrons and the electrons assigned to each atom. The sum should equal the total number of valence electrons for the molecule.
- Determine the formal charge for each atom by subtracting the number of owned electrons from the number of valence electrons. A positive formal charge means that the atom has lost electrons, while a negative formal charge means that it has gained electrons.
By following these steps, you can accurately determine the formal charge in a Lewis structure. This allows you to understand the distribution of electrons in a molecule and predict its chemical behavior.
Understanding the role of lone pairs in Lewis structures
In Lewis structures, lone pairs play a crucial role in determining the shape and reactivity of a molecule. Lone pairs are pairs of electrons that are not involved in bonding with other atoms. Instead, they reside on an atom and occupy a specific region of space around the nucleus. Lone pairs are represented in Lewis structures as pairs of dots.
Lone pairs have a significant impact on the overall geometry of a molecule. They exert a repulsive force on bonded electron pairs, pushing them away and influencing the molecule’s shape. This repulsion between lone pairs and bonded pairs, known as the lone pair effect, is crucial for understanding molecular geometry.
The presence of lone pairs can also affect the reactivity of a molecule. Lone pairs can act as Lewis bases, donating their electrons to form a new chemical bond. This can result in the formation of new molecular species or the initiation of various chemical reactions.
Furthermore, lone pairs can also participate in resonance structures, where they can move around within a molecule, changing its electronic distribution. This ability of lone pairs to participate in resonance greatly influences the stability and reactivity of certain molecules.
In summary, understanding the role of lone pairs in Lewis structures is essential for predicting the shape, reactivity, and stability of molecules. Lone pairs influence molecular geometry, participate in chemical reactions, and contribute to resonance structures, all of which ultimately determine the behavior and properties of a given compound.