
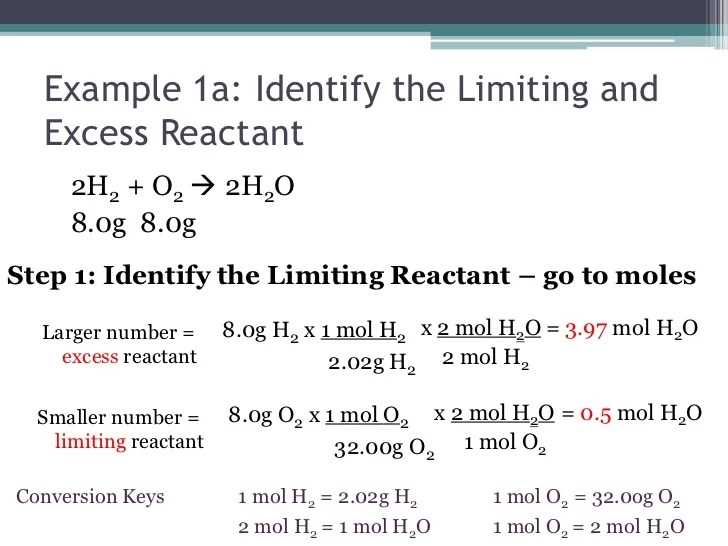
Understanding the concept of limiting and excess reactants is crucial in chemistry, as it allows scientists to determine the maximum amount of product that can be formed in a chemical reaction. To aid in this understanding, many educational resources provide worksheets with answers in PDF format.
These worksheets not only test students’ knowledge on limiting and excess reactants but also provide detailed explanations and step-by-step solutions. By using these worksheets, students can practice various problems to solidify their understanding and enhance their problem-solving skills.
The answers provided in the PDFs show the correct approach and methodology to solving the problems. Students can compare their own answers to the provided ones to identify any errors or misconceptions they may have. This feedback allows them to learn from their mistakes and make improvements for future problem-solving.
Additionally, the PDF format allows for easy accessibility and printing. Students can conveniently download the worksheets and answers on their computers or mobile devices, allowing them to study and practice anywhere, anytime. Moreover, the printable format enables students to work through the problems at their own pace and annotate as needed.
In conclusion, the availability of limiting and excess reactants worksheet answers in PDF format is an essential resource for students studying chemistry. It provides comprehensive explanations, step-by-step solutions, and the opportunity for self-assessment. By utilizing these worksheets, students can enhance their understanding, improve their problem-solving skills, and achieve success in their chemistry studies.
Limiting and Excess Reactants Worksheet Answers PDF
In the field of chemistry, it is important to understand the concept of limiting and excess reactants in a chemical reaction. A limiting reactant is the substance that is completely consumed in a reaction, while an excess reactant is the substance that is left over after the reaction is complete. It is crucial to identify the limiting reactant in order to accurately determine the amount of product that can be produced.
The limiting reactant can be determined by comparing the mole ratios of the reactants in the balanced chemical equation. By calculating the moles of each reactant and comparing their ratios, it is possible to identify which reactant will be completely consumed first, thus limiting the amount of product that can be formed. The excess reactant, on the other hand, will have some remaining amount after the reaction is complete.
The limiting and excess reactants worksheet provides practice problems for students to apply their understanding of this concept. The worksheet typically includes a series of chemical reactions with reactant amounts given, and the students are required to determine the limiting and excess reactants, as well as calculate the amount of product that can be formed. The worksheet often includes step-by-step solutions and explanations to assist students in their learning process.
By utilizing the limiting and excess reactants worksheet answers PDF, students can check their work and verify the accuracy of their calculations. This PDF file provides the complete solutions to the worksheet, allowing students to compare their answers and identify any errors or misunderstandings. The detailed explanations provided in the answers guide students through the thought process and methodology involved in solving these types of problems.
What is a Limiting Reactant?

If you have ever cooked a recipe that requires a specific ratio of ingredients, then you have already encountered the concept of a limiting reactant. In chemistry, a limiting reactant is a substance that limits the amount of product that can be formed in a chemical reaction. It is the reactant that is completely consumed during the reaction, while the other reactants may be left over.
Let’s consider an example to understand this concept better. Imagine you are making sandwiches and you have 8 slices of bread, 4 slices of cheese, and 2 slices of ham. Each sandwich requires 2 slices of bread, 1 slice of cheese, and 1 slice of ham. In this scenario, the limiting reactant would be the ham because you only have enough ham to make 2 sandwiches, even though you have enough bread and cheese to make 4 sandwiches.
In chemistry, determining the limiting reactant is important because it allows us to calculate the maximum amount of product that can be obtained from a given set of reactants. This information is crucial for efficient production processes and can help chemists optimize reactions to achieve higher yields.
To determine the limiting reactant, one must compare the stoichiometric ratios of the reactants and their actual quantities. Whichever reactant has the smallest stoichiometric ratio compared to its available quantity will be the limiting reactant.
Understanding the concept of limiting reactants is crucial in chemistry as it helps us predict and control the outcome of chemical reactions. By identifying the limiting reactant, chemists can optimize reaction conditions and ensure the most efficient use of resources.
How to Determine the Limiting Reactant
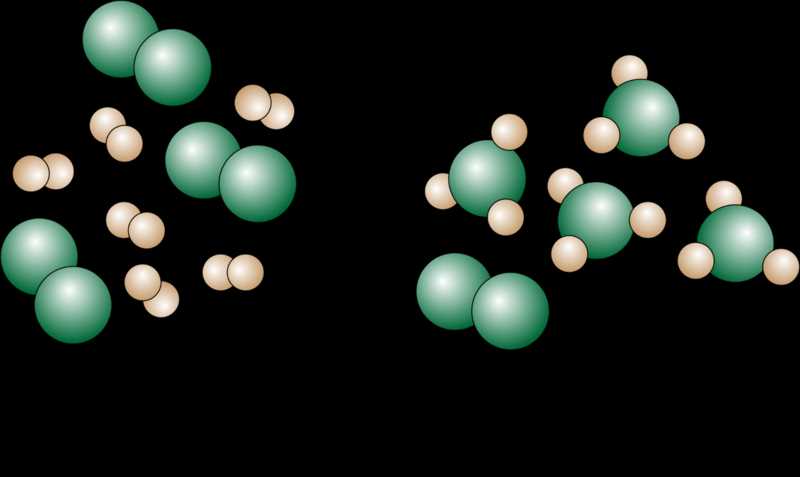
When performing a chemical reaction, it is important to know which reactant will be completely consumed first. This reactant is called the limiting reactant. To determine the limiting reactant, one must compare the number of moles of each reactant to the stoichiometry of the balanced chemical equation.
To begin, one must determine the number of moles of each reactant. This can be done by using the given masses of the reactants and their molecular weights. The molecular weight of a substance can be found by adding up the atomic weights of all the elements in the chemical formula.
After determining the number of moles of each reactant, one must then compare these values to the stoichiometry of the balanced chemical equation. The stoichiometry is the ratio of the coefficients of the reactants in the chemical equation. For example, if the balanced equation is 2A + 3B → C, the stoichiometry would be 2:3.
Once the stoichiometry is known, it can be used to calculate the theoretical yield of the product for each reactant. The reactant that produces the smallest theoretical yield is the limiting reactant.
By identifying the limiting reactant, scientists can determine the maximum amount of product that can be formed in a reaction. This information is crucial for optimizing reaction conditions and understanding the efficiency of a chemical process.
Calculation Example for Finding the Limiting Reactant
In a chemical reaction, the limiting reactant is the one that gets completely consumed and determines the amount of product that can be formed. To find the limiting reactant, you need to compare the amounts of each reactant and calculate the amount of product that can be formed from each.
Let’s consider an example. Suppose we have a reaction between hydrogen gas (H2) and oxygen gas (O2) to form water (H2O). The balanced chemical equation for this reaction is:
2H2 + O2 → 2H2O
Now let’s say we have 4 moles of hydrogen gas and 3 moles of oxygen gas. To find the limiting reactant, we need to calculate the amount of product that can be formed from each reactant. We can do this by using stoichiometry, which relates the ratios of reactants and products in a chemical equation.
Using the stoichiometry, we can calculate that 4 moles of hydrogen gas can produce 8 moles of water. Similarly, 3 moles of oxygen gas can produce 6 moles of water. Since the balanced equation shows that 2 moles of water are produced for every mole of oxygen gas, we can see that the oxygen gas is the limiting reactant in this case.
By determining the limiting reactant, we can calculate the theoretical yield of the product. In this example, the theoretical yield of water would be 6 moles, which is the amount that can be obtained if all the oxygen gas reacts completely.
What is an Excess Reactant?
An excess reactant, also known as a excess reagent, is a reactant in a chemical reaction that is present in a greater quantity than what is required for the reaction to go to completion. In other words, it is the reactant that is left over after the reaction has reached equilibrium.
During a chemical reaction, reactants are combined in specific proportions based on the balanced equation. However, in many cases, one reactant may be added in excess to ensure that the other reactant is completely consumed. This is done to maximize the efficiency of the reaction and to ensure that the desired product is obtained in the highest yield possible.
The excess reactant is not completely consumed in the reaction and remains in the reaction mixture. It does not contribute to the formation of the desired product, and its presence is often undesirable as it can lead to waste and inefficiency in the reaction process.
Identifying the excess reactant is important in determining the limiting reactant, which is the reactant that is completely consumed and determines the maximum amount of product that can be obtained. By knowing the amount of excess reactant present, one can calculate the exact amount of limiting reactant required and optimize the reaction conditions.
In conclusion, an excess reactant is a reactant that is present in a greater quantity than what is required for a reaction. It does not contribute to the formation of the desired product and is often added to ensure the complete consumption of the other reactant.
How to Determine the Excess Reactant
When performing a chemical reaction, it is important to determine which reactant is in excess and which is limiting. The excess reactant is the reactant that is present in greater quantity than is necessary for the reaction to occur. By identifying the excess reactant, scientists can calculate the theoretical yield of the reaction.
The first step in determining the excess reactant is to write and balance the chemical equation for the reaction. This equation shows the stoichiometric relationship between the reactants and products. Once the equation is balanced, the coefficients can be used to determine the mole ratio between the reactants.
To find the excess reactant, one must compare the actual amounts of the reactants used in the reaction. This can be done by calculating the number of moles of each reactant used. The stoichiometry of the reaction can then be used to determine the number of moles of the other reactant that would be needed to completely react with the given amount of the first reactant.
By comparing the calculated amount of the second reactant to the actual amount used, it is possible to determine which reactant is in excess. The reactant that produces a smaller amount of product compared to the other reactant is the excess reactant. This reactant is not completely consumed in the reaction and will be left over after the reaction is complete.
It is important to identify the excess reactant because it affects the theoretical yield of the reaction. Theoretical yield is the maximum amount of product that can be obtained from a given amount of reactants, assuming complete reaction. By knowing the excess reactant, scientists can calculate the theoretical yield and compare it to the actual yield obtained in the reaction to determine the efficiency of the reaction.
Calculation Example for Finding the Excess Reactant

In chemical reactions, it is common for reactants to be present in excess or to be limited in quantity. The excess reactant is the substance that is not fully consumed in the reaction, while the limiting reactant is the substance that determines the maximum amount of product that can be formed.
Calculating the excess reactant involves comparing the amount of each reactant to the stoichiometric ratio given by the balanced chemical equation. Let’s consider an example:
Example:
Suppose we have a reaction between hydrogen gas (H2) and oxygen gas (O2) to form water (H2O). If we have 10 moles of hydrogen gas and 15 moles of oxygen gas, which reactant is in excess?
To determine the excess reactant, we need to compare the number of moles of each reactant to their stoichiometric ratio in the balanced equation. The balanced equation for the reaction is:
2 H2 + O2 -> 2 H2O
Based on the equation, for every 2 moles of hydrogen gas, we need 1 mole of oxygen gas. Therefore, if we have 10 moles of hydrogen gas, we would need 5 moles of oxygen gas for a 1:2 ratio.
In this case, we have 15 moles of oxygen gas, which is in excess compared to the stoichiometric ratio. The hydrogen gas is the limiting reactant since we don’t have enough oxygen gas to react with it fully.
Therefore, in this example, the excess reactant is oxygen gas and the limiting reactant is hydrogen gas.