When conducting chemical reactions, it is important to understand the concept of limiting reactants and percent yield. The limiting reactant is the reactant that is completely consumed in a chemical reaction, thus limiting the amount of product that can be formed. On the other hand, percent yield is a measure of how efficient a reaction is in producing the desired product.
In order to determine the limiting reactant, one must first calculate the number of moles of each reactant using their respective molar masses. Then, the mole ratio between the reactants and the product is used to determine which reactant will be consumed completely. The reactant that produces the smallest amount of product is the limiting reactant, as it limits the amount of product that can be formed.
Percent yield, on the other hand, is calculated by dividing the actual yield (the amount of product obtained in the reaction) by the theoretical yield (the maximum amount of product that can be obtained based on stoichiometric calculations), and then multiplying by 100. This gives a percentage that represents how efficiently the reaction produced the desired product.
By understanding the concepts of limiting reactants and percent yield, chemists can optimize reactions to maximize product formation and minimize waste. These concepts are essential in industrial processes, where efficiency and cost-effectiveness are key considerations. Additionally, they are important in laboratory settings, as accurate calculations and measurements are crucial for obtaining reliable results. Understanding the fundamentals of limiting reactants and percent yield allows chemists to make informed decisions and improve the overall efficiency of chemical processes.
What is a limiting reactant?

A limiting reactant, also known as a limiting reagent, refers to the substance that is completely consumed in a chemical reaction and limits the amount of product that can be formed. In other words, it is the reactant that is present in the smallest quantity, causing it to dictate the maximum amount of product that can be obtained. The concept of a limiting reactant is crucial in determining the actual yield of a reaction.
When multiple reactants are involved in a chemical reaction, it is important to identify the limiting reactant to accurately calculate the percent yield. This is done by comparing the stoichiometric coefficients of the reactants and their actual amounts. The reactant with the smallest stoichiometric coefficient-to-amount ratio will be the limiting reactant.
For example: Let’s consider the reaction between hydrogen gas (H2) and oxygen gas (O2) to form water (H2O). The balanced equation is:
- 2H2 + O2 → 2H2O
If we have 3 moles of H2 and 2 moles of O2, we can determine the limiting reactant as follows:
- Calculate the moles of product that can be obtained from each reactant using stoichiometry.
- Compare the moles of product to the actual moles of reactant.
- Identify the reactant with the smaller ratio of product moles to reactant moles as the limiting reactant.
In this case, if we calculate the moles of product, we find that 3 moles of H2 can produce 6 moles of H2O, while 2 moles of O2 can produce only 2 moles of H2O. Therefore, oxygen gas is the limiting reactant.
In conclusion, identifying the limiting reactant is crucial to determining the maximum amount of product that can be formed in a reaction. It allows for accurate calculations of the percent yield, which measures the efficiency of a chemical reaction.
How to determine the limiting reactant?
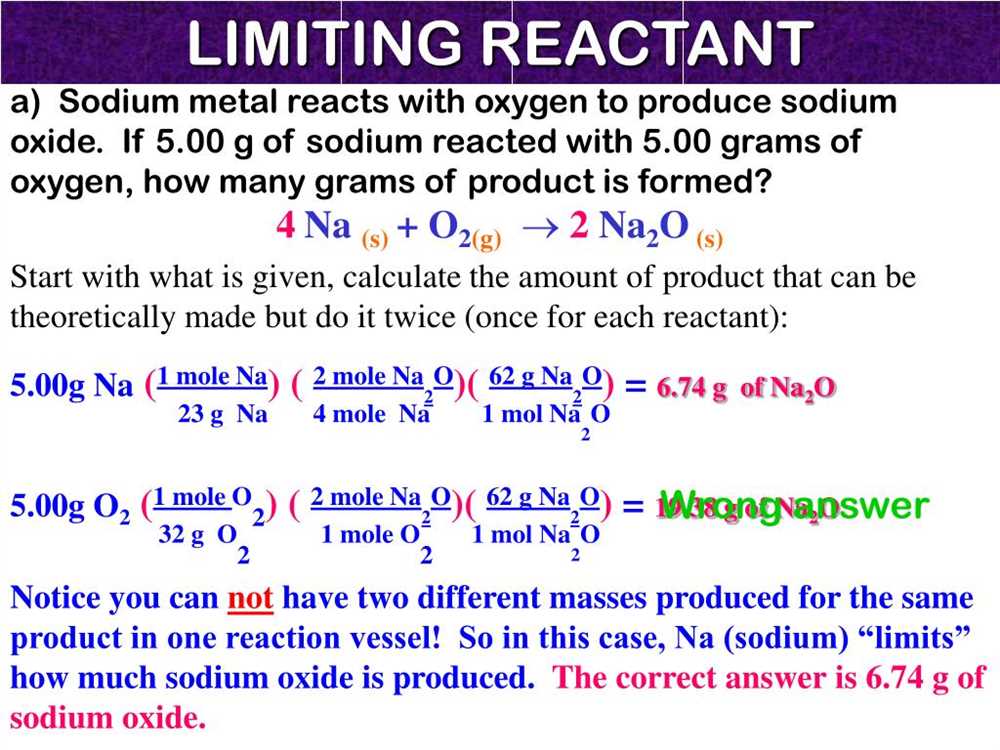
The limiting reactant is the reactant that is completely consumed in a chemical reaction, thereby limiting the amount of product that can be formed. To determine the limiting reactant, you need to compare the stoichiometry of the reactants and their quantities in the reaction.
Here are the steps to determine the limiting reactant:
- Write the balanced equation: Start by writing the balanced chemical equation for the reaction. This will give you the stoichiometry of the reactants and products.
- Convert the given quantities to moles: Convert the given quantities of the reactants from grams to moles, using the molar mass of each substance.
- Calculate the moles of product: Use the stoichiometry of the balanced equation to determine the moles of product that can be formed from each reactant.
- Compare the moles of product: Compare the moles of product calculated in the previous step for each reactant. The reactant that produces the smaller amount of product is the limiting reactant.
Once you have determined the limiting reactant, you can use it to calculate the theoretical yield of the product. The theoretical yield is the maximum amount of product that can be formed based on the stoichiometry of the reaction and the limiting reactant. The percent yield is calculated by dividing the actual yield by the theoretical yield and multiplying by 100.
In conclusion, determining the limiting reactant is crucial in understanding the efficiency of a chemical reaction and calculating the theoretical and percent yields of the product.
What is percent yield?
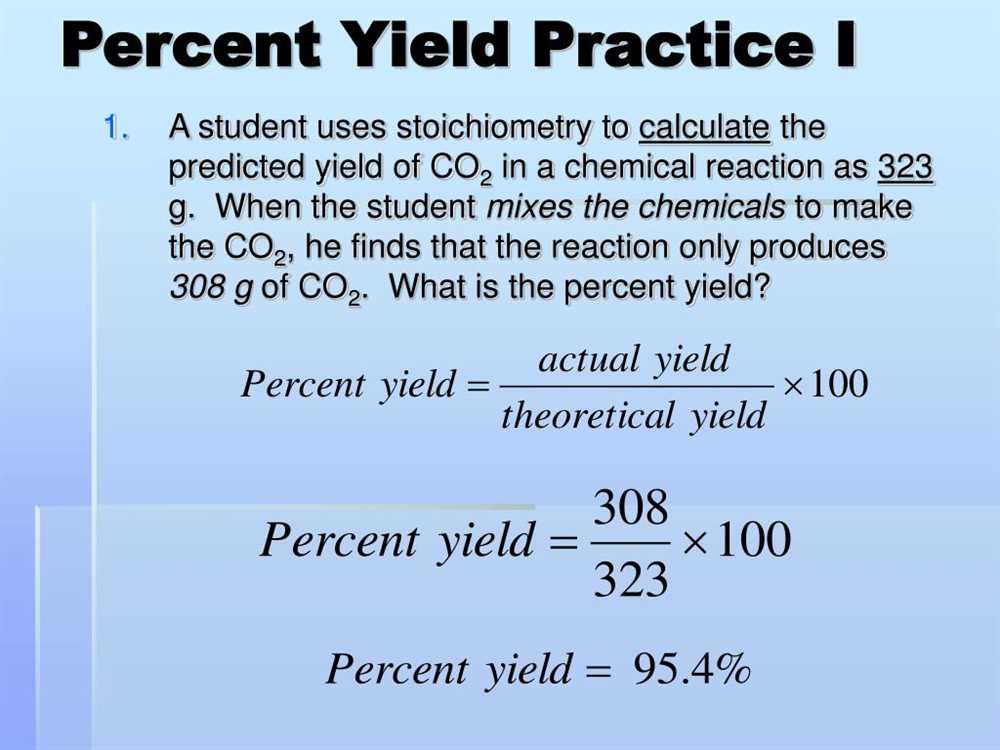
Percent yield is a concept used in chemistry to measure the efficiency of a chemical reaction by comparing the actual amount of product obtained to the theoretical yield, which is the maximum possible amount of product that could be obtained.
To calculate percent yield, the actual yield (the amount of product obtained in the reaction) is divided by the theoretical yield, and then multiplied by 100 to express it as a percentage. The formula for percent yield is:
- Percent Yield = (Actual Yield / Theoretical Yield) × 100
Percent yield is an important measure because it indicates how successful a reaction was in converting reactants into products. A high percent yield indicates that the reaction was efficient and produced a significant amount of product, while a low percent yield suggests that the reaction may have been incomplete or inefficient.
Various factors can affect the percent yield of a reaction, such as the purity of the reactants, the stoichiometry of the reaction, and any losses or side reactions that may occur. By calculating percent yield, chemists can assess the effectiveness of a reaction and make adjustments to improve its efficiency.
How to Calculate Percent Yield?
Calculating percent yield is an essential step in analyzing chemical reactions. It allows us to determine the efficiency of a reaction and assess how much product was actually obtained compared to the maximum amount theoretically possible. The percent yield is calculated by comparing the actual yield, which is the amount of product obtained experimentally, to the theoretical yield, which is the amount of product calculated based on stoichiometry.
To calculate percent yield, follow these steps:
- Identify the limiting reactant in the reaction. The limiting reactant is the one that gets completely consumed and determines the maximum amount of product that can be formed.
- Calculate the theoretical yield by using stoichiometric calculations. This involves finding the mole ratios between the limiting reactant and the product, and then converting moles to grams if necessary.
- Perform the reaction in the laboratory and obtain the actual yield. This is usually measured by weighing the product obtained.
- Divide the actual yield by the theoretical yield and multiply by 100 to get the percent yield. The formula is:
Percent Yield = (Actual Yield / Theoretical Yield) * 100
It is important to note that percent yield can never be greater than 100%. This is because it represents the efficiency of the reaction, and it is not possible to obtain more product than the maximum amount that can be produced based on stoichiometry. Percent yield values lower than 100% indicate that the reaction did not proceed to completion or that there were losses during the experimental process.
Calculating percent yield is a fundamental skill in chemistry and allows scientists to evaluate the success of reactions and optimize their conditions for better efficiency.
Factors Affecting Percent Yield

Percent yield is a measure of how efficiently a chemical reaction produces the desired product. There are several factors that can affect the percent yield of a reaction:
- Reaction conditions: The conditions under which a reaction takes place, such as temperature and pressure, can significantly impact the percent yield. Different reactions may require specific conditions to achieve optimal yield. For example, a reaction that requires high temperature may result in a lower percent yield if the temperature is not maintained properly.
- Purity of reactants: The purity of the reactants used in a reaction can affect the percent yield. Impurities in the reactants can interfere with the reaction, resulting in a lower yield. Using high-quality, pure reactants can help improve the percent yield.
- Stoichiometry: The stoichiometric ratio of the reactants plays a crucial role in determining the percent yield. If the reactants are not present in the correct ratio, one of them may become the limiting reactant, leading to an incomplete reaction and lower yield.
- Efficiency of the reaction: The efficiency of the reaction itself can impact the percent yield. Some reactions may be prone to side reactions or other issues that reduce the yield. Optimizing the reaction conditions and carefully monitoring the reaction can help improve the efficiency and ultimately the percent yield.
By carefully considering and controlling these factors, chemists can work to maximize the percent yield in a reaction. This is important not only for practical reasons, such as reducing waste and costs, but also for understanding the fundamental principles behind the reaction and improving overall chemical knowledge.
Why is percent yield important?
The percent yield is an important concept in chemistry as it measures the efficiency of a chemical reaction. It represents the ratio of the actual yield to the theoretical yield, expressed as a percentage. The theoretical yield is the amount of product that would be obtained if the reaction were to go to completion according to the stoichiometry of the balanced equation. The actual yield is the amount of product obtained in a laboratory experiment.
By calculating and comparing the percent yield, chemists can evaluate the success of a reaction and determine its efficiency. A high percent yield indicates that the reaction went to completion and produced the expected amount of product, while a low percent yield suggests that there were factors that hindered the reaction or caused a loss of product.
Knowing the percent yield is important for several reasons. Firstly, it allows chemists to assess the feasibility of a reaction. If the percent yield is low, it may not be practical or economical to produce the desired product on a larger scale. Additionally, the percent yield provides insight into the quality and purity of the product. A high percent yield indicates that the product is likely to be pure, while a low percent yield suggests the presence of impurities or side reactions.
The percent yield also helps in troubleshooting and optimization of chemical processes. By analyzing the factors that affect the percent yield, scientists can identify and modify the conditions that lead to low yields. This knowledge can guide the development of more efficient and sustainable synthetic routes in pharmaceutical and industrial applications.
Overall, the percent yield is a crucial parameter in chemical reactions, providing valuable information about the efficiency, feasibility, and quality of a reaction. It allows chemists to evaluate and optimize reactions, ensuring the production of high-quality products with minimal waste.
Examples of Limiting Reactant and Percent Yield Calculations
In chemistry, limiting reactant and percent yield calculations are used to determine the maximum amount of product that can be formed in a chemical reaction, as well as the efficiency of the reaction. These calculations are essential in understanding the overall yield and efficiency of a reaction.
One example of a limiting reactant calculation involves the reaction between hydrogen gas (H2) and oxygen gas (O2) to produce water (H2O). The balanced chemical equation for this reaction is:
2H2 + O2 → 2H2O
In this reaction, if there is an excess of hydrogen gas and a limited amount of oxygen gas, the oxygen gas would be the limiting reactant. This means that the amount of water formed would be limited by the amount of oxygen gas available. To calculate the maximum amount of water that can be formed, one can use stoichiometry and the molar ratios provided in the balanced equation.
Another example of a percent yield calculation involves the reaction between iron (Fe) and sulfur (S) to produce iron(II) sulfide (FeS). The balanced equation for this reaction is:
Fe + S → FeS
If a chemist performs this reaction in the lab and obtains 8 grams of iron(II) sulfide, but the theoretical yield (calculated using stoichiometry) is 10 grams, the percent yield can be calculated by dividing the actual yield (8 grams) by the theoretical yield (10 grams) and multiplying by 100. The percent yield in this case would be 80%.
These are just a few examples of how limiting reactant and percent yield calculations are used in chemistry. These calculations are crucial for understanding and predicting the outcomes of chemical reactions, as well as determining the efficiency of a reaction.
Practice problems with limiting reactant and percent yield worksheet answers

When studying chemical reactions, it is important to understand the concept of a limiting reactant, which is the reactant that is completely used up in a reaction, thus limiting the amount of product that can be formed. To help students practice this concept, teachers often provide worksheets with practice problems.
These worksheets typically include balanced chemical equations and ask students to determine the limiting reactant and calculate the percent yield of the reaction. The answers to these practice problems are essential for students to check their understanding and improve their problem-solving skills.
For example, one practice problem may present a balanced chemical equation and ask students to identify the limiting reactant from a given amount of two reactants. In this case, students need to calculate the moles of each reactant, determine the mole ratio between the reactants based on the balanced equation, and identify the reactant that produces fewer moles of the product.
Another common type of practice problem involves calculating the percent yield of a reaction. Students are given the theoretical yield, which is the maximum amount of product that can be obtained based on the balanced equation, and the actual yield, which is the amount of product obtained in the laboratory. By dividing the actual yield by the theoretical yield and multiplying by 100, students can calculate the percent yield.
These practice problems with limiting reactant and percent yield worksheet answers allow students to reinforce their understanding of these concepts and develop their problem-solving skills. By practicing these calculations, students can become more confident in their ability to analyze chemical reactions and make accurate predictions about the amount of product that will be formed.