
When it comes to chemical reactions, the concept of limiting reagent plays a crucial role in determining the amount of products that can be formed. In this worksheet, we will dive deeper into the concept of limiting reagent and its application in stoichiometry.
A limiting reagent is a reactant that is completely consumed in a chemical reaction, thus limiting the amount of product that can be formed. It is the reactant that is present in the smallest stoichiometric amount compared to the other reactants involved in the reaction. In other words, it is the reactant that determines the maximum amount of product that can be obtained.
This worksheet provides a series of problems that require identifying the limiting reagent, calculating the amount of product formed, and determining the amount of excess reactants leftover. By solving these problems, students will not only strengthen their understanding of stoichiometry, but also enhance their problem-solving skills in chemistry.
With the answers provided in this worksheet, students can compare their solutions and check their understanding of the concept of limiting reagent. This enables them to identify any misconceptions and reinforce their knowledge of stoichiometry. By practicing these problems, students will gain confidence in their ability to apply the concept of limiting reagent to real-world scenarios.
What is a limiting reagent?
In chemistry, a limiting reagent is a reactant that is completely consumed in a chemical reaction. It determines the maximum amount of product that can be formed based on its stoichiometry and the amount of other reactants present. The limiting reagent is often referred to as the “bottleneck” of the reaction, as it restricts the amount of product that can be formed.
To understand the concept of a limiting reagent, it is important to consider the stoichiometry of the reaction. Stoichiometry is the quantitative relationship between the amounts of reactants and products in a chemical reaction. It is based on the balanced chemical equation, which shows the ratio of reactants and products.
When performing a stoichiometric calculation, it is necessary to identify the limiting reagent. This is done by comparing the amount of each reactant present with the stoichiometric ratio in the balanced chemical equation. The reactant with the smaller stoichiometric ratio is the limiting reagent.
Once the limiting reagent is identified, it can be used to determine the maximum amount of product that can be formed. This is done by calculating the theoretical yield, which is the amount of product that would be obtained if all of the limiting reagent reacted completely.
In summary, a limiting reagent is a reactant that is completely consumed in a chemical reaction. It determines the maximum amount of product that can be formed and is identified by comparing the amounts of reactants with the stoichiometric ratio in the balanced chemical equation.
Definition of a limiting reagent
A limiting reagent, also known as a limiting reactant, is a chemical substance that is completely consumed in a chemical reaction, thereby limiting the amount of product that can be formed. In other words, the limiting reagent is the reactant that is present in the lowest stoichiometric amount compared to the other reactants.
The concept of a limiting reagent is crucial in stoichiometry, which is the branch of chemistry that deals with the quantitative relationships between reactants and products in a chemical reaction. By identifying the limiting reagent, chemists can determine the maximum amount of product that can be obtained from a given amount of reactants.
To determine the limiting reagent, one must first write a balanced chemical equation for the reaction. Then, the amount of each reactant is compared to the stoichiometric coefficients in the equation. The reactant that is present in the smallest amount, compared to its stoichiometric coefficient, is the limiting reagent.
The limiting reagent is crucial in determining the theoretical yield of a reaction, which is the maximum amount of product that can be obtained in an ideal scenario. It is also used to calculate percent yield, which is the actual yield of product obtained in a specific experiment compared to the theoretical yield.
Understanding the concept of a limiting reagent is important in various fields, such as pharmaceuticals, materials science, and environmental chemistry, as it allows chemists to optimize reaction conditions and maximize the efficiency of chemical processes.
The Importance of Identifying the Limiting Reagent
In a chemical reaction, the limiting reagent is the reactant that is completely consumed, limiting the amount of product that can be formed. Identifying the limiting reagent is crucial in determining the maximum amount of product that can be obtained and helps optimize the reaction conditions.
One of the main reasons for identifying the limiting reagent is to avoid wastage of resources. By knowing which reactant is going to be consumed first, we can ensure that we use the appropriate amounts of reactants. This prevents excess reactants from being wasted and helps reduce the cost of the reaction.
Furthermore, the identification of the limiting reagent allows us to calculate the theoretical yield of the reaction. The theoretical yield is the maximum amount of product that can be obtained according to the stoichiometry of the reaction. By knowing the limiting reagent, we can determine the amount of product that can be formed and compare it to the actual yield, which allows us to evaluate the efficiency of the reaction.
Identifying the limiting reagent is also important for safety reasons. In some reactions, the presence of excess reactants can lead to side reactions or the formation of unwanted byproducts. By ensuring that only the necessary amounts of reactants are used, we can minimize the risks associated with these side reactions and ensure a safer working environment.
Overall, identifying the limiting reagent is crucial for optimizing resources, calculating the theoretical yield, and ensuring safety in chemical reactions. It allows chemists to make informed decisions regarding reactant quantities, leading to more efficient and cost-effective processes.
How to identify the limiting reagent?
The limiting reagent, also known as the limiting reactant, is the reactant that is completely consumed in a chemical reaction, thereby limiting the amount of product that can be formed. Identifying the limiting reagent is crucial for determining the theoretical yield of a reaction.
To identify the limiting reagent, you need to compare the stoichiometry of the reactants with the moles of each reactant present. This can be done by following these steps:
- Determine the balanced chemical equation for the reaction.
- Convert the known quantities of each reactant to moles.
- Use the coefficients from the balanced equation to determine the mole ratio of the reactants.
- Compare the calculated mole ratios of the reactants to determine which one is in excess and which one is limiting.
The reactant with the smaller calculated mole ratio is the limiting reagent because it will be completely consumed before the other reactant. Once the limiting reagent is identified, you can use its mole ratio with the product to calculate the theoretical yield of the reaction.
It is important to note that the limiting reagent may not always be the reactant with the smaller given quantity. The stoichiometry of the reaction and the mole ratios must be taken into account to accurately identify the limiting reagent.
Step-by-step process to identify the limiting reagent
In order to determine the limiting reagent in a chemical reaction, you need to follow a step-by-step process. This process involves analyzing the stoichiometry of the reaction and comparing the amount of each reactant to determine which one will be completely consumed first.
- Write and balance the chemical equation: Start by writing the balanced chemical equation for the reaction. This equation represents the relationship between the reactants and the products. Ensure that the equation is balanced in terms of both atoms and charge.
- Convert the given quantities to moles: Convert the given quantities of each reactant to moles using their respective molar masses. This step allows you to compare the amounts of different substances on an equal basis.
- Determine the stoichiometry: Examine the coefficients in the balanced chemical equation to determine the ratio of moles between the reactants and products. This ratio will help you determine the amount of each reactant needed to completely react.
- Calculate the amount of product formed: Using the stoichiometry ratio, calculate the amount of product that would be formed if each reactant were to completely react. This step will allow you to determine which reactant is in excess and which one is limiting.
- Compare the calculated amount of product with the actual amount: Compare the calculated amount of product with the actual amount that was obtained in the reaction. The reactant that produces less product is the limiting reagent.
By following this step-by-step process, you can easily identify the limiting reagent in a chemical reaction. This information is crucial for determining the maximum amount of product that can be formed and for optimizing reaction conditions. It allows chemists to understand the efficiency of a reaction and make adjustments if necessary.
Sample limiting reagent worksheet
In chemistry, the limiting reagent is the reactant that determines the maximum amount of product that can be formed in a chemical reaction. To understand this concept better, let’s take a look at a sample limiting reagent worksheet.
Suppose we have a reaction where 2 moles of hydrogen gas (H2) react with 1 mole of oxygen gas (O2) to produce water (H2O). The first step is to determine the balanced chemical equation for this reaction:
- H2 + O2 → H2O
Now, let’s say we have 4 moles of hydrogen gas and 3 moles of oxygen gas. To determine the limiting reagent, we need to compare the moles of each reactant to the stoichiometric ratio in the balanced equation.
Since the stoichiometric ratio is 2:1 for hydrogen to oxygen, we would need 2 moles of hydrogen gas for every 1 mole of oxygen gas. In this case, since we have 4 moles of hydrogen gas and 3 moles of oxygen gas, hydrogen gas is in excess and oxygen gas is the limiting reagent.
Using this information, we can calculate the maximum amount of water that can be produced. Since oxygen gas is the limiting reagent, we use its moles to determine the moles of water formed. In this case, we would have 1.5 moles of water.
In conclusion, a sample limiting reagent worksheet helps us understand how to determine the limiting reagent in a chemical reaction and calculate the maximum amount of product that can be formed.
Limiting reagent worksheet problem 1
In this problem, we are given a balanced chemical equation and the amounts (in moles) of two reactants. The goal is to determine the limiting reagent and the theoretical yield of a product.
The balanced chemical equation for the reaction is:
2H2 + O2 → 2H2O
The amounts of the reactants are:
- H2: 2.5 moles
- O2: 3.2 moles
To determine the limiting reagent, we need to compare the moles of each reactant to their stoichiometric coefficients in the balanced equation. The stoichiometric coefficient of H2 is 2, while the coefficient of O2 is 1.
Using the given amounts, we calculate:
- H2: (2.5 moles) / (2) = 1.25 moles
- O2: (3.2 moles) / (1) = 3.2 moles
Based on these calculations, we can see that there is an excess of O2 (3.2 moles) compared to H2 (1.25 moles). Therefore, H2 is the limiting reagent.
Since each mole of H2 produces 2 moles of H2O, the theoretical yield of H2O can be calculated as:
(1.25 moles H2) × (2 moles H2O / 2 moles H2) = 1.25 moles H2O
Therefore, the theoretical yield of H2O in this reaction is 1.25 moles.
Limiting reagent worksheet problem 2

In this limiting reagent worksheet problem, we will analyze a chemical reaction and determine which reagent is the limiting reagent and the amount of product that can be formed.
The given reaction is: 2HCl + Na2CO3 → 2NaCl + H2O + CO2
To solve this problem, we will compare the amount of each reagent given and calculate the amount of product that can be formed based on the limiting reagent.
Let’s say we have 10 moles of HCl and 8 moles of Na2CO3. To determine the limiting reagent, we need to convert the moles of each reagent to moles of the product formed. Since the stoichiometric ratio between HCl and Na2CO3 is 2:1, we need to divide the moles of each reagent by their respective coefficients.
HCl: 10 moles HCl ÷ 2 = 5 moles of product formed
Na2CO3: 8 moles Na2CO3 ÷ 1 = 8 moles of product formed
From the calculated values, we can see that HCl is the limiting reagent because it produces less moles of product compared to Na2CO3. Therefore, the maximum amount of product that can be formed is 5 moles.
In conclusion, in this limiting reagent worksheet problem 2, we have determined that HCl is the limiting reagent and the maximum amount of product that can be formed is 5 moles.
Limiting Reagent Worksheet Problem 3
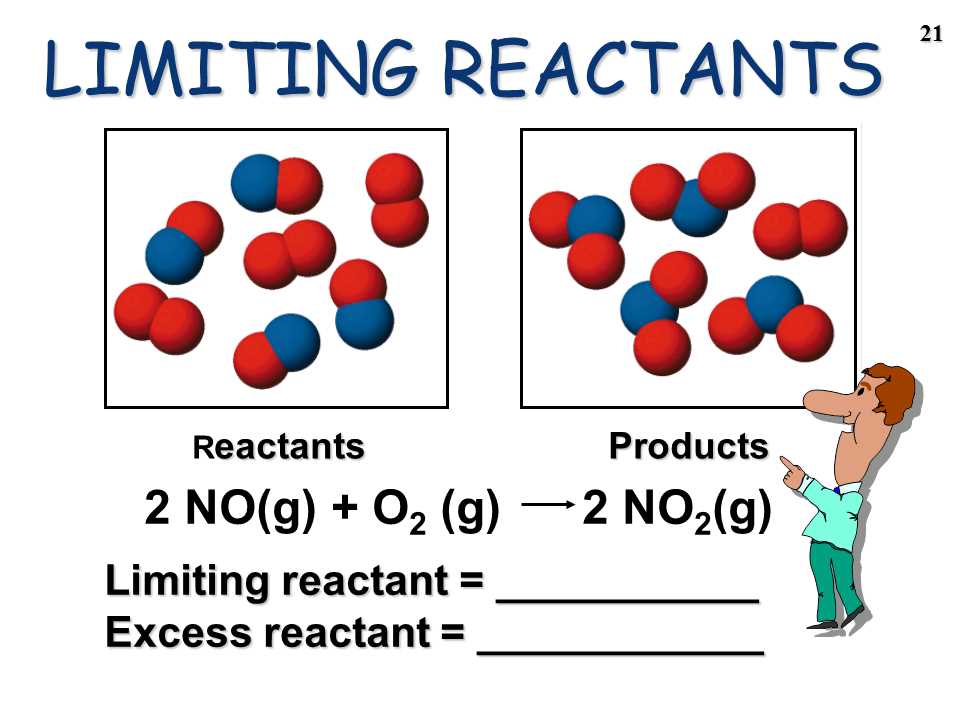
In this problem, we will analyze a chemical reaction to determine the limiting reagent. The reaction we will consider is the combination of copper sulfate (CuSO4) and iron (Fe) to form iron sulfate (FeSO4) and copper (Cu). The balanced equation for this reaction is:
CuSO4 + Fe → FeSO4 + Cu
To determine the limiting reagent, we need to compare the amount of each reactant to the stoichiometry of the reaction. We are given that we have 10 grams of CuSO4 and 6 grams of Fe. We will calculate the moles of each reactant and then compare the moles to the stoichiometric ratio of the reaction.
First, let’s calculate the moles of CuSO4:
- Molar mass of CuSO4 = 63.55 g/mol (Cu) + 32.07 g/mol (S) + 4 * 16.00 g/mol (O) = 159.61 g/mol
- Moles of CuSO4 = 10 g / 159.61 g/mol = 0.0627 mol
Next, let’s calculate the moles of Fe:
- Molar mass of Fe = 55.85 g/mol
- Moles of Fe = 6 g / 55.85 g/mol = 0.1074 mol
Now, let’s compare the moles of each reactant to the stoichiometric ratio of the reaction:
- CuSO4 : Fe = 1 : 1
- 0.0627 mol : 0.1074 mol ≈ 0.582 : 1
From this comparison, we can see that CuSO4 is the limiting reagent because its moles are less than the stoichiometric ratio with Fe. This means that all of the CuSO4 will be consumed in the reaction and there will be excess Fe left over.
In conclusion, in the given reaction, CuSO4 is the limiting reagent. This information is important in determining the maximum amount of product that can be formed and for calculating the theoretical yield.