In the study of physics, understanding the concepts of matter and thermal energy is crucial. Matter refers to anything that has mass and takes up space, while thermal energy is the energy associated with the movement of particles within a substance. To test your knowledge and assess your understanding of these concepts, a worksheet with questions and answers can be a valuable tool.
The matter and thermal energy worksheet answers provide detailed explanations and solutions to the questions presented in the worksheet. They cover a range of topics such as the properties of matter, states of matter, changes in states of matter, and the transfer of thermal energy. By reviewing these answers, you can solidify your understanding of these fundamental concepts.
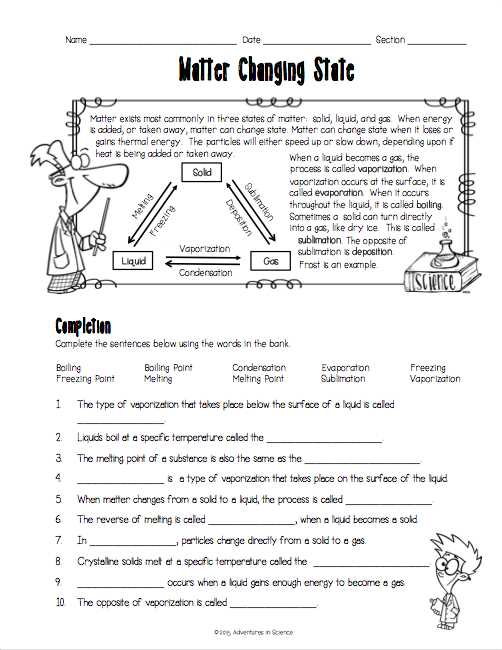
For example, one question on the worksheet might ask about the difference between solids, liquids, and gases. The answer would explain that solids have a definite shape and volume, while liquids have a definite volume but no fixed shape, and gases have neither a definite shape nor volume. It would also mention that these differences are due to the arrangement and movement of particles in each state of matter.
Another question might inquire about how thermal energy is transferred. The answer would discuss the three main methods of heat transfer: conduction, convection, and radiation. It would explain that conduction occurs through direct contact between particles, convection involves the movement of particles within a fluid, and radiation involves the emission and absorption of electromagnetic waves.
Overall, the matter and thermal energy worksheet answers serve as a valuable resource for students learning about these fundamental concepts. By studying and comprehending these answers, you can enhance your knowledge and excel in the study of physics.
Matter and Thermal Energy Worksheet Answers
In the study of matter and thermal energy, it is important to understand the relationship between the two. Matter refers to anything that has mass and takes up space, while thermal energy is the energy that comes from the random movement of particles within matter. In this worksheet, we will explore various questions and concepts related to matter and thermal energy.
1. What is the difference between heat and temperature?
Heat and temperature are often used interchangeably, but they are actually two different concepts. Heat refers to the transfer of energy from a warmer object to a cooler object due to a temperature difference. Temperature, on the other hand, is a measure of the average kinetic energy of the particles in a substance. Essentially, heat is the transfer of thermal energy, while temperature is a measure of the amount of thermal energy.
2. How does thermal energy affect the states of matter?
Thermal energy plays a crucial role in determining the states of matter. As thermal energy increases, the particles within matter gain more kinetic energy and move faster. In solids, the particles vibrate in fixed positions. As thermal energy increases, the particles start to move more freely, leading to a phase change from solid to liquid. Further increase in thermal energy causes the particles to move even more freely, resulting in a phase change from liquid to gas. The opposite happens when thermal energy decreases, causing a phase change from gas to liquid and then from liquid to solid.
3. How does the transfer of thermal energy occur?
Thermal energy can be transferred through three main processes: conduction, convection, and radiation. Conduction occurs when thermal energy is transferred through direct contact between particles. Convection involves the transfer of thermal energy through the movement of particles in a fluid (liquid or gas). Radiation refers to the transfer of thermal energy through electromagnetic waves, such as heat from the sun reaching the Earth. These three processes are important for understanding how thermal energy is distributed and transferred within matter.
- Matter and thermal energy
- Heat and temperature
- States of matter and thermal energy
- Transfer of thermal energy
Understanding Matter and Thermal Energy
In order to fully understand the concept of matter and thermal energy, it is important to have a clear understanding of what these terms mean. Matter refers to anything that has mass and takes up space. This can include solids, liquids, and gases. Thermal energy, on the other hand, is the energy that comes from the motion of particles in matter. It is often associated with heat and can be transferred from one object to another through processes such as conduction, convection, and radiation.
Thermal energy is closely related to temperature. Temperature is a measure of the average kinetic energy of the particles in a substance. The higher the temperature, the more kinetic energy the particles have, and therefore, the more thermal energy is present. This explains why objects feel hotter when their temperature is higher.
In addition to temperature, the amount of matter present also affects the thermal energy. The more matter there is, the more particles there are to have kinetic energy, and hence, the more thermal energy is present. This is why larger objects often have more thermal energy than smaller objects, even if their temperatures are the same.
Understanding matter and thermal energy is crucial in various fields, including physics, chemistry, and engineering. It allows scientists and engineers to study how heat is transferred and how it affects different materials. This knowledge can be applied in various industries, such as designing efficient cooling systems, developing energy-efficient buildings, and improving the efficiency of engines. Overall, understanding matter and thermal energy provides a foundation for understanding the behavior of matter and the processes that occur in our everyday lives.
Types of Matter
Matter is anything that has mass and takes up space. It exists in various forms, each with its own properties and characteristics. In this article, we will explore the different types of matter and their unique features.
1. Elements
An element is a type of matter that cannot be broken down into simpler substances through chemical methods. It consists of atoms, which are the basic building blocks of matter. Each element is defined by the number of protons in its nucleus. For example, hydrogen is the simplest element with only one proton, while gold has 79 protons.
2. Compounds

A compound is a substance made up of two or more different elements that are chemically combined. Unlike mixtures, the elements in compounds are bonded together in fixed ratios. For instance, water is a compound made up of two hydrogen atoms and one oxygen atom. Compounds have unique properties that are different from the elements that make them up.
3. Mixtures
A mixture is a combination of two or more substances that are physically blended together. Unlike compounds, the substances in mixtures do not chemically react with each other. Mixtures can be homogeneous, where the components are evenly distributed (like salt dissolved in water), or heterogeneous, where the components are not evenly distributed (like oil and water). Mixtures can be separated through physical methods, such as filtration or distillation.
4. Solutions
A solution is a special type of mixture in which one substance (called the solute) is dissolved in another substance (called the solvent). Solutions are usually homogeneous, with the solute particles evenly distributed throughout the solvent. Common examples of solutions include saltwater (solute: salt, solvent: water) and carbonated beverages (solute: carbon dioxide, solvent: water).
5. Colloids
Colloids are mixtures in which microscopic particles are dispersed in a medium, creating a cloudy or milky appearance. Unlike solutions, the particles in colloids do not settle out over time. Examples of colloids include milk (with particles of fat suspended in water) and fog (with water droplets suspended in air).
- Summary: Matter comes in different types, including elements, compounds, mixtures, solutions, and colloids. Each type has its own unique properties and characteristics, which can affect their behavior and interactions with other substances. Understanding these different types of matter helps scientists classify and study the world around us.
Properties of Matter
Matter is everything around us, and it is important to understand its properties in order to study and analyze its behavior. Matter is made up of tiny particles called atoms, which are the building blocks of all substances. The properties of matter can be divided into two main categories: physical properties and chemical properties.
Physical properties are characteristics that can be observed or measured without changing the composition of the substance. These properties include mass, volume, density, color, texture, and conductivity. Mass refers to the amount of matter in an object, while volume is the amount of space it occupies. Density is the ratio of mass to volume, and it can determine whether an object sinks or floats in a fluid. Color is the visual perception of the reflected light, while texture refers to the feel or consistency of a substance. Conductivity is the ability of a material to conduct electricity or heat.
Chemical properties, on the other hand, describe how a substance interacts with other substances and undergoes chemical changes. These properties are typically observed through chemical reactions. Examples of chemical properties include flammability, reactivity, and toxicity. Flammability refers to the ability of a substance to burn, while reactivity describes its tendency to react with other substances. Toxicity refers to the ability of a substance to cause harm or damage to living organisms.
In conclusion, understanding the properties of matter is essential for understanding how it behaves and interacts with other substances. By studying and analyzing these properties, scientists can make predictions and develop new materials for various applications.
The Relation Between Matter and Thermal Energy
Thermal energy is the energy that is created by the movement of particles within a substance. It is directly related to the amount of matter present, as the more particles there are in a substance, the more thermal energy it will possess. This relationship can be explained by the concept of temperature.
Temperature is a measure of the average kinetic energy of particles in a substance. When the particles in a substance are moving faster, they have more kinetic energy, and therefore the substance has a higher temperature. The transfer of thermal energy occurs when there is a difference in temperature between two substances. The particles in the substance with higher temperature will transfer their kinetic energy to the particles in the substance with lower temperature, resulting in a transfer of thermal energy.
Example: If you place a hot cup of coffee on a table, the thermal energy from the hot liquid will transfer to the particles in the table, eventually reaching a state of equilibrium where both the coffee and the table have the same temperature. The transfer of thermal energy is also responsible for processes such as conduction, convection, and radiation.
It is important to note that the amount of thermal energy transferred does not depend on the size or shape of the substances involved, but rather on the temperature difference between them. This means that even though a large object may have more particles and therefore more thermal energy, it will not necessarily transfer more thermal energy to a smaller object if the temperature difference is not significant.
In conclusion, the relation between matter and thermal energy is evident through the concept of temperature and the transfer of thermal energy. The amount of matter present in a substance directly affects the amount of thermal energy it possesses, and the transfer of thermal energy occurs when there is a difference in temperature between substances.
Measuring Thermal Energy
Thermal energy, also known as heat energy, is a form of energy that results from the motion of particles within a substance. It is important to be able to measure thermal energy in order to understand and study its effects. There are several ways to measure thermal energy, each with its own advantages and limitations.
Thermometers are commonly used to measure thermal energy. They work by utilizing the expansion and contraction of a liquid or gas to determine the temperature of an object or substance. Thermometers can be calibrated to display the temperature in various units, such as Celsius, Fahrenheit, or Kelvin. They are widely used in everyday life, from measuring body temperature to monitoring the temperature in a laboratory.
Calorimeters are another tool used to measure thermal energy. They work based on the principle of conservation of energy, where the heat gained or lost by a substance is equal to the heat gained or lost by its surroundings. Calorimeters typically consist of an insulated container, a known amount of water, and a thermometer. By measuring the change in temperature of the water before and after a reaction or process, the amount of thermal energy involved can be calculated. Calorimeters are commonly used in chemistry experiments to determine the heat of reactions.
- Infrared thermography is a non-contact method of measuring thermal energy. It uses specialized cameras that detect and measure infrared radiation emitted by objects. The cameras produce images called thermograms, which represent the temperature distribution of a surface. This technique is often used in building inspections, electrical inspections, and medical imaging.
- Specific heat capacity is a property of a substance that measures its ability to store thermal energy. It is defined as the amount of heat required to raise the temperature of a unit mass of the substance by one degree Celsius. By knowing the specific heat capacity of a substance, the amount of thermal energy it contains can be calculated using the equation Q = m × c × ΔT, where Q is the thermal energy, m is the mass of the substance, c is the specific heat capacity, and ΔT is the change in temperature.
These are just a few examples of the methods used to measure thermal energy. Each method has its own advantages and limitations, and the choice of method depends on the specific situation and requirements. By accurately measuring thermal energy, scientists and engineers can better understand its behavior and make informed decisions in various fields, from energy conservation to material processing.