In science and engineering, accurate measurement of volume is crucial for conducting experiments and ensuring precision in calculations. The metric system provides a standardized set of units for measuring volume, making it easier to communicate and compare results. In this lab, students learn about metric measurement of volume and practice using various tools and techniques.
One of the main tools used in this lab is the graduated cylinder, a cylindrical container with calibrated markings. Students carefully pour liquid into the graduated cylinder and read the measurement at the meniscus, the curved surface of the liquid caused by capillary action. This avoids parallax errors and ensures accurate readings. Another tool used is the burette, which has a valve at the bottom for precise control of liquid flow. Students use the burette to measure the volume of liquids dispensed during experiments.
During the lab, students also learn about the concept of displacement as a method for measuring volume. By placing solid objects into a container filled with water, students can measure the increase in volume caused by the object. This technique is useful for irregularly shaped objects that cannot be measured directly using a graduated cylinder or burette. Students record the amount of water displaced and calculate the volume of the object using the principle of fluid displacement.
After conducting the lab, students compare their measurements with the known values provided in the answer key. This allows them to assess their accuracy and identify any sources of error. By analyzing the sources of error, students can refine their measurement techniques and improve their overall understanding of metric measurement of volume. This lab provides a hands-on experience for students to develop their skills and gain confidence in using metric measurement tools.
Metric Measurement Volume Lab Answer Key
In the metric measurement volume lab, students were tasked with accurately measuring the volume of various objects using metric units. The answer key provides the correct measurements for each object and serves as a reference for students to check their work.
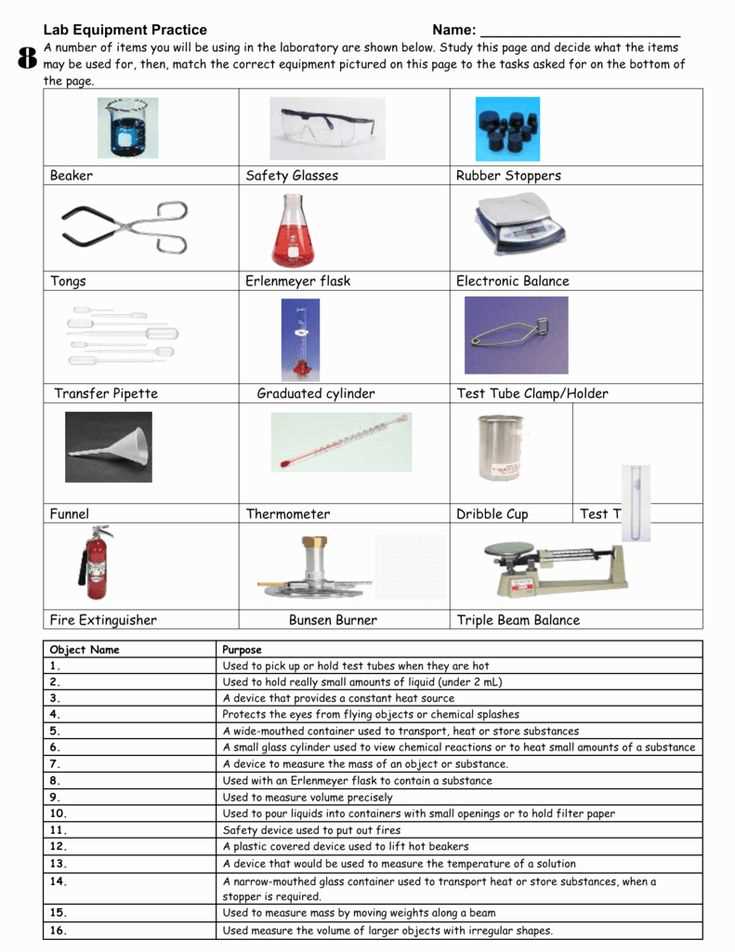
Object 1: Beaker
The volume of the beaker was measured using the metric unit of milliliters. The correct measurement for the beaker is 250 mL.
Object 2: Block of Wood
The volume of the block of wood was measured using the metric unit of cubic centimeters (cm^3). The correct measurement for the block of wood is 100 cm^3.
Object 3: Glass Cup
The volume of the glass cup was also measured in milliliters. The correct measurement for the glass cup is 350 mL.
Object 4: Plastic Bottle
The volume of the plastic bottle was measured in liters. The correct measurement for the plastic bottle is 1.5 L.
Object 5: Metal Sphere
The volume of the metal sphere was measured using the metric unit of cubic centimeters (cm^3). The correct measurement for the metal sphere is 200 cm^3.
Object 6: Graduated Cylinder
The volume of the graduated cylinder was measured in milliliters. The correct measurement for the graduated cylinder is 75 mL.
Object 7: Empty Test Tube
The volume of the empty test tube was also measured in milliliters. The correct measurement for the empty test tube is 10 mL.
By comparing their measurements to the answer key, students can determine if they accurately measured the volume of each object. This allows them to practice and improve their skills in metric measurement.
Background Information on Metric Measurement
Metric measurement is a system of measurement used around the world that is based on powers of ten. It was first established in France in the late 18th century and has since become the standard system of measurement in most countries. The system is based on the meter, which is defined as the distance light travels in a vacuum in 1/299,792,458 of a second. This provides a consistent and universal standard for measurement.
The metric system is composed of basic units for length, mass, and volume, as well as derived units for other quantities. The basic unit of length is the meter, the basic unit of mass is the kilogram, and the basic unit of volume is the liter. These units can be combined and converted using prefixes to represent larger or smaller quantities. For example, the kilometer is equal to 1000 meters, and the milliliter is equal to 1/1000 of a liter.
Measurement in the metric system is generally more straightforward and convenient than in other systems, such as the imperial system used in the United States. This is because the metric system is based on logical and consistent units, making calculations and conversions easier. Additionally, the use of decimal notation in the metric system allows for simple and efficient arithmetic.
In summary, metric measurement is a system of measurement based on powers of ten, with the meter, kilogram, and liter as the basic units for length, mass, and volume. The system provides a universal standard and is known for its consistency and ease of use compared to other systems. Understanding and using metric measurement is essential for accurate and precise scientific measurements.
The Importance of Volume Measurement in the Laboratory
The accurate measurement of volume is a crucial aspect of laboratory work across various scientific disciplines. Whether it is in biology, chemistry, or physics, precise volume measurements are essential for conducting experiments, analyzing data, and ensuring the validity of the results obtained. In the laboratory, volume measurements are used to determine the amount of a substance, the concentration of a solution, and to perform various calculations and conversions.
Reliable and accurate volume measurements are vital for the reproducibility and reliability of scientific experiments. Without proper volume measurements, scientists would not be able to confidently compare and replicate experiments, leading to increased uncertainty and decreased confidence in the results. Precise volume measurements also help to ensure that the correct amount of reagents or substances is used, preventing errors that could potentially affect the outcome of an experiment.
Volume measurements are particularly important in chemistry. In chemical reactions, the stoichiometry, or the ratio of reactants and products, plays a crucial role. The volume of a substance can be used to determine its mass, concentration, or density, which are key parameters in chemical calculations. Additionally, accurate volume measurements are necessary for preparing solutions with the desired concentration, as even slight deviations can significantly affect the outcome of a reaction or analysis.
- Tools such as graduated cylinders, pipettes, and burettes are commonly used in the laboratory for volume measurements.
- Proper technique and calibration of these instruments are essential to ensure accurate measurements. Regular calibration and maintenance of laboratory equipment help to minimize systematic errors and ensure reliable results.
- Volume measurements also play a crucial role in quality control and assurance. In industries such as pharmaceuticals and manufacturing, accurate volume measurements are essential to ensure the consistent production of products and adherence to regulations.
In conclusion, volume measurement is of utmost importance in the laboratory to ensure the accuracy and reliability of scientific experiments. With precise volume measurements, scientists can confidently analyze data, compare results, and draw meaningful conclusions. Proper technique, calibration, and the use of reliable measurement tools are key in obtaining accurate volume measurements, which are essential in various scientific disciplines.
Lab Procedure for Measuring Volume
In the lab, measuring volume accurately is crucial for many experiments and calculations. To ensure accurate measurements, the following procedure can be followed:
Materials Needed:
- Graduated cylinder
- Beaker
- Pipette
- Water
Procedure:
- Place the graduated cylinder on a level surface.
- Pour the liquid you want to measure into the graduated cylinder. Make sure to pour slowly and avoid spilling.
- Read the volume of the liquid by looking at the meniscus, the curved surface of the liquid, at eye level. The volume reading should be taken at the bottom of the meniscus.
- Record the volume measurement in milliliters (mL).
- If the liquid level is above the desired volume, use a pipette to remove small amounts of liquid until the desired volume is reached.
- If the liquid level is below the desired volume, use a beaker to add small amounts of liquid until the desired volume is reached.
- For more precise measurements, use a pipette to add or remove small amounts of liquid as necessary.
- Repeat the measurement and recording process for any additional liquids or samples.
By following this lab procedure, accurate volume measurements can be obtained, ensuring reliable data for experiments and calculations.
Equipment used for Measuring Volume in the Lab
When conducting experiments in a laboratory, accurately measuring volume is crucial. Various equipment is used to ensure precise measurements of liquid volume. The following are some common instruments used for measuring volume in the lab:
- Graduated Cylinder: This tall, narrow container has markings along its side, indicating volume. It is typically used for measuring liquids with a high degree of accuracy. The volume is read at the meniscus, the curve at the top of the liquid.
- Volumetric Flask: A volumetric flask is a flat-bottomed glass container with a long neck and a stopper. It is used for preparing solutions and holds a specific volume at a particular temperature. The volume of the liquid it contains is accurate to within ±0.05 mL.
- Pipette: A pipette is a narrow tube used to transfer a specific quantity of liquid from one container to another. It usually has either a fixed or adjustable volume, allowing for precise measurements.
Besides these instruments, there are other tools used for measuring volume in the lab, such as the burette, syringe, and beaker. Each instrument has its own advantages and is selected based on the requirements of the experiment. Accurate volume measurements are key to obtaining reliable data in various scientific fields, from chemistry to biology and beyond.
Common Errors and Challenges in Volume Measurement
Accurate volume measurement is essential in many scientific experiments and industrial processes. However, there are several common errors and challenges that can affect the precision and accuracy of volume measurements.
1. Meniscus Reading Errors
One common error in volume measurement is reading the meniscus incorrectly. The meniscus is the curved surface of a liquid in a container, and the volume should be read at the bottom of the meniscus. When the eye is not at the same level as the meniscus, parallax errors can occur, leading to inaccurate readings. To avoid this error, it is crucial to position the eye at the same level as the meniscus and ensure proper lighting during reading.
2. Uncalibrated or Inaccurate Equipment
Another challenge in volume measurement is using uncalibrated or inaccurate equipment. Graduated cylinders, pipettes, and burettes should be calibrated regularly to ensure their accuracy. If the equipment is not properly calibrated, it can lead to significant measurement errors. It is important to follow the manufacturer’s guidelines for calibrating and maintaining the equipment to obtain reliable volume measurements.
3. Temperature Effects on Volume
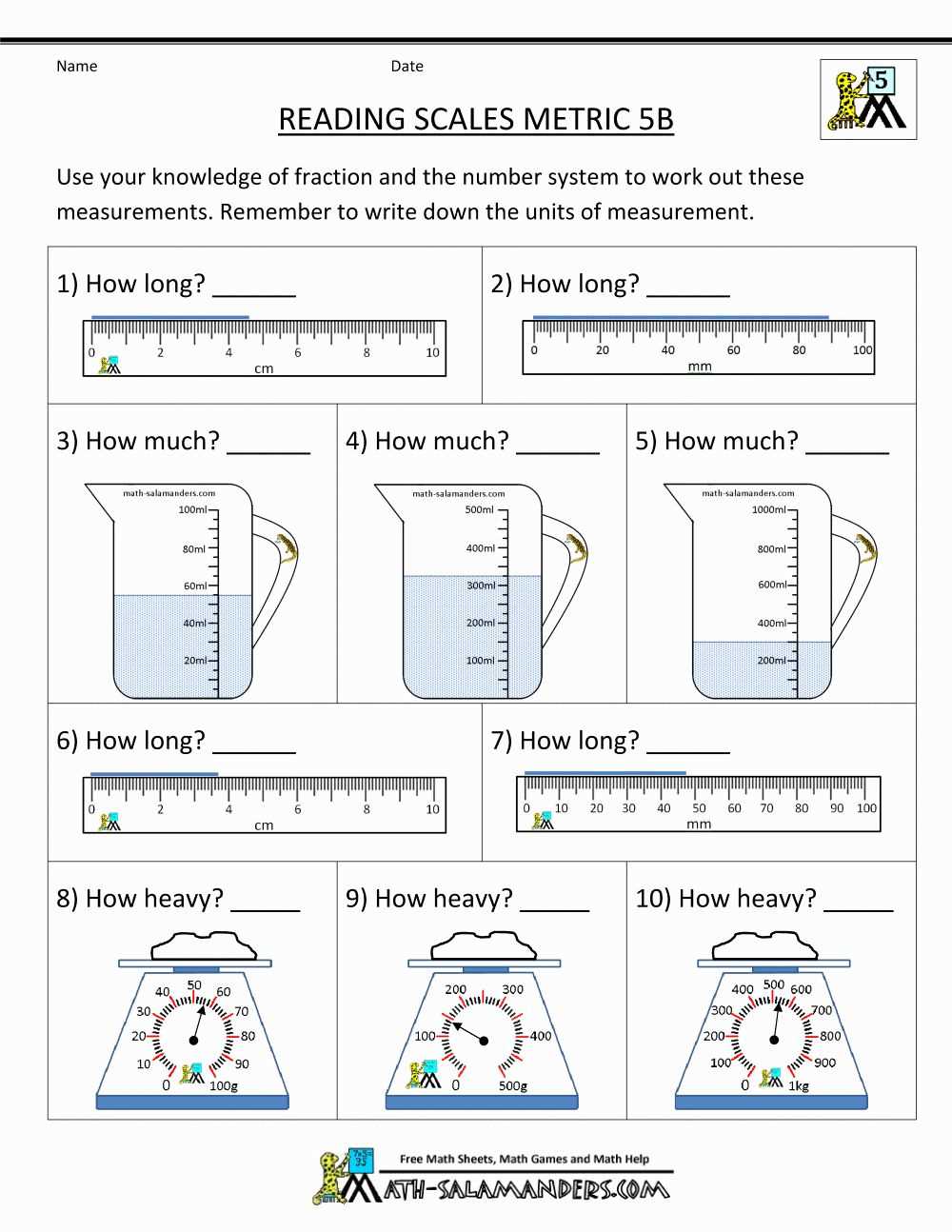
The volume of a liquid can be influenced by temperature changes. The expansion or contraction of a liquid with temperature can lead to errors in volume measurement. It is crucial to take into account the temperature of the substance being measured and make appropriate corrections using temperature conversion factors, especially when dealing with volatile liquids or when measurements need to be compared across different temperatures.
4. Air Bubbles or Contaminants
Air bubbles or contaminants in a liquid can affect the accuracy of volume measurements. Air bubbles can displace the liquid, leading to incorrect volume readings, while contaminants can alter the density and composition of the liquid, affecting its volume. To minimize errors, it is important to remove any air bubbles and ensure the cleanliness of the liquid and the container before making volume measurements.
In conclusion, volume measurement can be subject to various errors and challenges, including meniscus reading errors, uncalibrated or inaccurate equipment, temperature effects, and the presence of air bubbles or contaminants. Being aware of these common errors and taking appropriate precautions can help improve the accuracy and reliability of volume measurements in scientific and industrial settings.
Tips and Tricks for Accurate Volume Measurement
Accurate volume measurement is crucial in various fields, such as industry, scientific research, and healthcare. The precision of volume measurements directly impacts the quality of products, experimental outcomes, and patient care. To ensure accurate volume measurement, it is essential to follow certain tips and tricks:
1. Use the appropriate measuring instrument:
Selecting the right measuring instrument is vital for accurate volume measurement. Graduated cylinders, burettes, pipettes, and syringes are commonly used tools for measuring volumes. Each instrument has its own precision level and range. Read the manufacturer’s guidelines to understand the instrument’s specifications and choose accordingly.
2. Maintain proper calibration:
Regularly calibrating the measuring instrument is crucial for maintaining accuracy. Over time, instruments may develop a slight error, affecting the volume measurements. Be sure to follow the calibration schedule recommended by the manufacturer or consult with a calibration service provider for accurate measurements.
3. Pay attention to meniscus:
When measuring liquid volumes in containers with narrow openings, such as graduated cylinders or pipettes, always read the measurement at the bottom of the meniscus. The meniscus refers to the curved surface of a liquid caused by capillary action. Reading at the bottom of the meniscus helps to reduce parallax errors and ensures accurate volume measurement.
4. Practice proper technique:

Mastering proper technique is essential for accurate volume measurement. Avoid touching the inside of the measuring instrument with fingers as the presence of oils can affect the accuracy. Ensure that the instrument is placed on a level surface to prevent tilting. Always use a clean and dry instrument for measuring volumes to prevent contamination or dilution.
5. Take multiple measurements:
To increase accuracy and reduce the chances of errors, take multiple measurements of the same volume. Taking the average of these measurements can help to minimize any systematic errors and provide a more reliable result.
By following these tips and tricks, you can improve the accuracy of volume measurements and ensure reliable results in your work or experiments.