
Gas laws play a crucial role in understanding the behavior of gases and their properties. These laws, which include Boyle’s Law, Charles’s Law, and Avogadro’s Law, allow us to predict how changing variables affect the pressure, volume, and temperature of a gas sample. To thoroughly grasp these concepts, practice is key. That’s where a mixed gas laws worksheet comes in handy.
The mixed gas laws worksheet is a valuable tool for students studying the principles of gas laws. It presents a series of problems that require the application of various gas laws to find the desired solution. With this worksheet, students can test their understanding and problem-solving skills in a structured and systematic way.
By providing step-by-step solutions and answers, the mixed gas laws worksheet helps students navigate through complex calculations and gain confidence in their ability to solve gas law problems. It offers a comprehensive review of the gas laws, allowing students to reinforce their knowledge and identify areas for improvement.
Ultimately, the mixed gas laws worksheet acts as a powerful learning aid, enabling students to enhance their conceptual understanding of gas laws and develop proficiency in applying these principles to real-world scenarios. With its practical exercises and answers, the worksheet serves as a valuable resource for students and educators alike, ensuring a solid foundation in the study of gas laws.
Mixed Gas Laws Worksheet Answers

Here are the answers to the mixed gas laws worksheet:
1. A sample of nitrogen gas occupies a volume of 500 mL at a pressure of 2 atm. If the temperature is decreased from 300 K to 200 K while the volume remains constant, what will be the new pressure?
To solve this problem, we can use the combined gas law equation: P1T1 = P2T2. Given that P1 = 2 atm, T1 = 300 K, and T2 = 200 K (while the volume remains constant), we can plug in these values and solve for P2: P2 = (P1 * T2) / T1. Plugging in the values, we get P2 = (2 * 200) / 300 = 1.33 atm.
2. A sample of oxygen gas has a volume of 2 L at a pressure of 3 atm. If the pressure is increased to 4 atm while the volume remains constant, what will be the new temperature?
Again, we can use the combined gas law equation to solve this problem: P1T1 = P2T2. Given that P1 = 3 atm, T1 is unknown, P2 = 4 atm, and the volume remains constant, we can solve for T2: T2 = (P1 * T1) / P2. Plugging in the values, we get T2 = (3 * T1) / 4. Since the volume remains constant, the temperature changes proportionally to the pressure, so T2 = (3 * T1) / 4 = 3/4 * T1. Therefore, the new temperature will be 3/4 of the original temperature.
3. A sample of carbon dioxide gas occupies a volume of 2 L at a temperature of 300 K. If the volume is increased to 4 L while the temperature remains constant, what will be the new pressure?
To solve this problem, we can use Boyle’s law equation: P1V1 = P2V2. Given that P1 is unknown, V1 = 2 L, P2 is unknown, V2 = 4 L, and the temperature remains constant, we can solve for P1 and P2: P1 = (P2 * V2) / V1 and P2 = (P1 * V1) / V2. Since the temperature remains constant, P1 and P2 are inversely proportional to the volume. Therefore, if the volume is doubled, the pressure will be halved: P2 = (P1 * 2) / 4 = P1 / 2.
- Question 1: The new pressure will be 1.33 atm.
- Question 2: The new temperature will be 3/4 of the original temperature.
- Question 3: The new pressure will be half of the original pressure.
These are the answers to the mixed gas laws worksheet. Make sure to double-check your calculations and understanding of the gas laws to ensure accuracy.
What are gas laws?
Gas laws are a set of mathematical relationships between the physical properties of gases. These laws describe how gases behave under different conditions of temperature, pressure, and volume.
One of the fundamental gas laws is Boyle’s law, which states that the volume of a gas is inversely proportional to its pressure, assuming the temperature remains constant. This means that as the pressure on a gas increases, its volume decreases, and vice versa.
Another important gas law is Charles’s law, which states that the volume of a gas is directly proportional to its temperature, assuming the pressure remains constant. This means that as the temperature of a gas increases, its volume also increases, and vice versa.
Combined, Boyle’s law and Charles’s law form the basis of the ideal gas law, which describes the behavior of an ideal gas under any set of conditions. The ideal gas law combines the relationships between pressure, volume, temperature, and the number of gas molecules present.
Gas laws are crucial in understanding many phenomena in chemistry and physics. They help scientists predict and explain the behavior of gases in various situations, such as the expansion and contraction of gases, the changes in pressure and volume during chemical reactions, and the behavior of gases at different temperatures and pressures.
Importance of understanding mixed gas laws
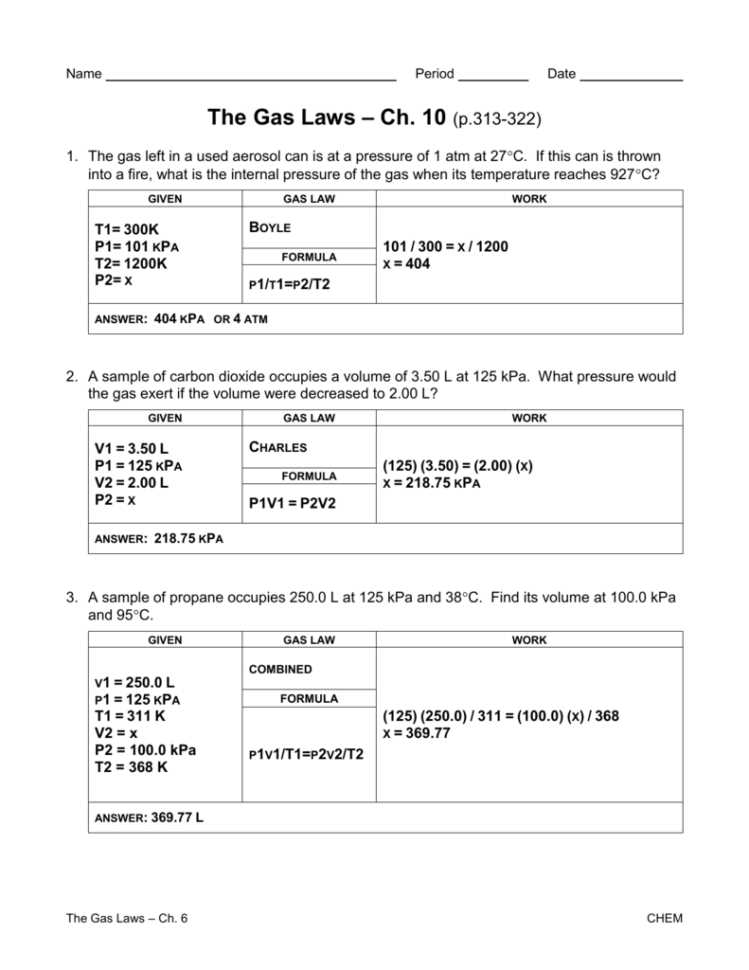
The understanding of mixed gas laws is of great importance in various fields ranging from chemistry to engineering. These laws help us to predict the behavior of gases when they are mixed together under different conditions. By understanding these laws, scientists and engineers can make accurate calculations and predictions, allowing for the design of efficient systems and processes.
One of the key concepts in mixed gas laws is the relationship between pressure, volume, and temperature. The ideal gas law, which incorporates these variables, allows us to calculate the properties of a gas mixture. This knowledge is essential in the design and operation of systems that involve the handling and transportation of gases, such as chemical plants, refrigeration systems, and compressed air systems.
For example: Let’s consider a scenario where a gas mixture needs to be compressed and stored in a container. Understanding the mixed gas laws allows us to determine the appropriate pressure and temperature conditions for the compression process, ensuring the safety and efficiency of the system. Without this knowledge, the containers could be overpressurized, leading to potential hazards or inefficiencies.
Another area where understanding mixed gas laws is crucial is in scientific research. Researchers often work with gas mixtures in laboratories, and accurate measurements and calculations are necessary for their experiments and analyses. Understanding mixed gas laws allows scientists to control and manipulate the properties of gas mixtures, enabling them to study the behavior of gases under specific conditions.
In conclusion, understanding mixed gas laws is vital for various applications in fields such as chemistry, engineering, and scientific research. It allows for accurate calculations and predictions, ensuring the safety and efficiency of systems involving gas mixtures. This knowledge is essential for designing and operating systems that handle gases and for conducting scientific experiments effectively.
Boyle’s Law
Boyle’s Law is one of the fundamental gas laws that describes the relationship between the pressure and volume of a gas sample, assuming constant temperature. It states that as the volume of a gas decreases, the pressure increases proportionally, and vice versa.
This law is named after Robert Boyle, an Irish scientist who first stated the relationship in the 17th century. Boyle’s experiments involved varying the volume of a gas sample while keeping the temperature constant and measuring the corresponding pressure changes. He observed that the product of pressure and volume remained constant.
Mathematically, Boyle’s Law can be represented as:
Pressure × Volume = Constant
or
P1V1 = P2V2
Where P1 and V1 are the initial pressure and volume, and P2 and V2 are the final pressure and volume.
Boyle’s Law is applicable to ideal gases under conditions of constant temperature. It helps in understanding various phenomena, such as the compression of gases, the operation of pumps, and even the functioning of the human lungs.
In summary, Boyle’s Law states that as the volume of a gas decreases, the pressure increases, and as the volume increases, the pressure decreases, as long as the temperature remains constant. This law provides a basis for understanding the behavior of gases and is widely used in various scientific and industrial applications.
Charles’s Law
Charles’s Law, also known as the law of volumes, states that when the temperature of a gas increases, its volume also increases, provided that the pressure remains constant. This relationship is expressed by the equation V1/T1 = V2/T2, where V1 and V2 represent the initial and final volumes of the gas, and T1 and T2 represent the initial and final temperatures, respectively.
This law is based on the observation that gases tend to expand when heated. As the temperature increases, the particles in the gas move faster and collide more frequently with each other and the walls of the container. This increased collision rate leads to an increase in the volume of the gas.
Example:
Let’s say we have a balloon filled with helium gas at a temperature of 25 °C (298 K). If we heat the balloon to a temperature of 50 °C (323 K) while keeping the pressure constant, according to Charles’s Law, the volume of the balloon will increase proportionally. If the initial volume of the balloon is 2 liters, the final volume can be calculated using the equation V1/T1 = V2/T2:
- Initial volume (V1) = 2 liters
- Initial temperature (T1) = 298 K
- Final temperature (T2) = 323 K
Using the equation, we can calculate the final volume (V2):
V2 = (V1 x T2) / T1 = (2 x 323) / 298 ≈ 2.18 liters
Therefore, according to Charles’s Law, the volume of the balloon will increase to approximately 2.18 liters when heated to 50 °C.
Gay-Lussac’s Law
Gay-Lussac’s Law, also known as the pressure-temperature law, states that the pressure of a gas is directly proportional to its temperature, as long as the volume and amount of gas remain constant. This law is named after the French chemist Joseph Louis Gay-Lussac, who first observed this relationship in the early 19th century.
According to Gay-Lussac’s Law, when the temperature of a gas increases, the pressure of the gas also increases, and vice versa. This can be explained by the kinetic theory of gases, which states that the temperature of a gas is directly related to the average kinetic energy of its molecules. As the temperature increases, the molecules move faster and collide with the walls of the container more frequently, resulting in an increase in pressure.
Mathematically, Gay-Lussac’s Law can be expressed as:
P1/T1 = P2/T2
Where P1 and T1 are the initial pressure and temperature of the gas, and P2 and T2 are the final pressure and temperature, respectively. This equation shows that the ratio of pressure to temperature remains constant, assuming the volume and amount of gas are constant.
Gay-Lussac’s Law is particularly useful in understanding the behavior of gases at different temperatures and pressures. It has important applications in various fields, including industrial processes, weather forecasting, and chemistry. By understanding the relationship between pressure and temperature, scientists can make predictions and calculations to optimize processes and ensure safety in various applications.
Avogadro’s Law
Avogadro’s Law is a fundamental principle in the field of gas laws. It states that equal volumes of gases at the same temperature and pressure contain an equal number of molecules. In other words, the number of molecules in a gas is directly proportional to its volume.
This law was named after the Italian scientist Amedeo Avogadro, who proposed it in 1811. Avogadro’s Law is based on the concept that gases are made up of individual molecules that are in constant motion. The volume occupied by a gas depends on the number of gas molecules present, rather than the nature of the gas itself.
An important implication of Avogadro’s Law is that gases with the same volume and temperature will have the same number of molecules, regardless of their chemical identity. This principle allows us to compare and measure the quantities of different gases in a systematic way.
For example, if we have two gases at the same temperature and pressure, but different volumes, according to Avogadro’s Law, the gas with the larger volume will contain more molecules. This law is essential for understanding and predicting the behavior of gases in various physical and chemical processes.
Avogadro’s Law is closely related to other gas laws, such as Boyle’s Law and Charles’s Law, which describe the relationships between pressure, volume, and temperature of a gas. Together, these laws form the basis for the study of gas behavior and are widely used in fields like chemistry, physics, and engineering.
Combined Gas Law
The combined gas law is a mathematical relationship between the pressure, volume, and temperature of a gas. It is derived from the individual gas laws, namely Boyle’s law, Charles’s law, and Gay-Lussac’s law.
The combined gas law can be expressed as:
P1V1/T1 = P2V2/T2
- P1 and P2 represent the initial and final pressures of the gas, measured in units of pressure such as atmospheres (atm) or pascals (Pa).
- V1 and V2 represent the initial and final volumes of the gas, measured in units of volume such as liters (L) or cubic meters (m3).
- T1 and T2 represent the initial and final temperatures of the gas, measured in units of temperature such as Kelvin (K) or Celsius (°C).
This equation allows us to calculate the changes in pressure, volume, and temperature of a gas when the other two variables are known. It is particularly useful when dealing with scenarios where multiple variables change simultaneously.
For example, if we know the initial and final volumes and temperatures of a gas, we can use the combined gas law to calculate the final pressure. Similarly, if we know the initial and final pressures and volumes, we can calculate the final temperature.
By understanding the combined gas law, scientists and engineers can manipulate the conditions of a gas system to achieve desired outcomes, such as optimizing chemical reactions or designing efficient gas storage systems.