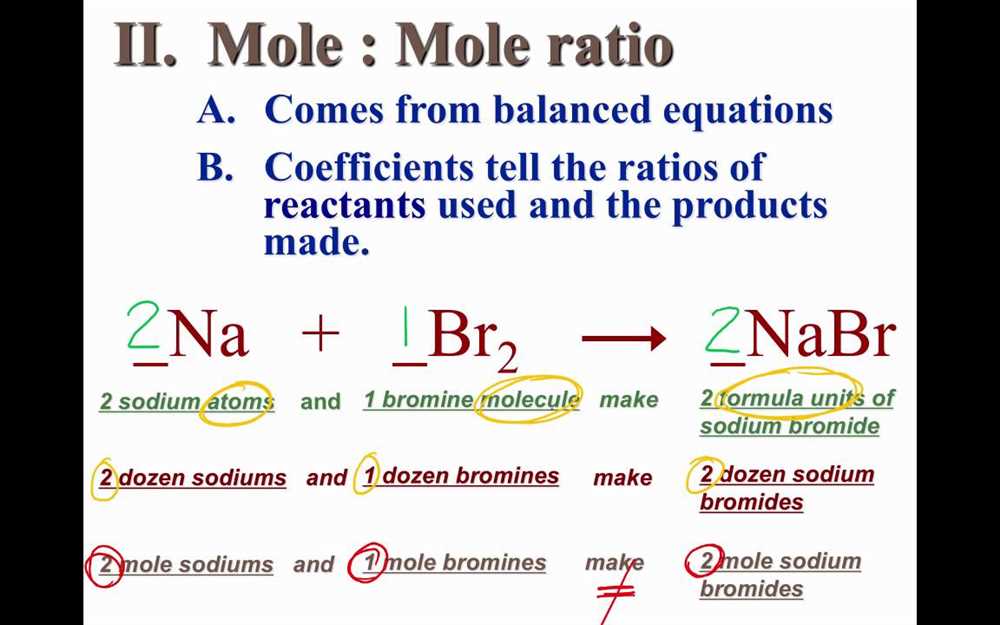
Chemistry is a fascinating subject that delves into the intricate world of atoms and molecules. One important concept in chemistry is mole ratios, which play a crucial role in understanding chemical reactions and stoichiometry. Mole ratios help us determine the quantities of reactants and products involved in a chemical equation and provide a basis for calculations.
The Mole Ratio Pogil activity is a collaborative learning exercise designed to enhance students’ understanding of mole ratios. By working through a series of guided questions and performing calculations, students gain a deeper insight into the relationship between reactants and products in a chemical reaction. To aid in their comprehension, a Mole Ratio Pogil Answer Key is provided, which serves as a valuable resource for students to check their work and reinforce their understanding.
Using the Mole Ratio Pogil Answer Key, students can verify their answers and determine if they have grasped the concept of mole ratios correctly. This key provides step-by-step solutions and explanations for each question, allowing students to identify any misconceptions or errors in their reasoning. The answer key not only facilitates correct answers but also encourages students to learn from their mistakes, promoting a deeper understanding of mole ratios and their application in stoichiometric calculations.
By utilizing the Mole Ratio Pogil Answer Key, students can enhance their problem-solving skills and develop a solid foundation in understanding the concept of mole ratios. This valuable resource empowers students to independently assess their progress and make adjustments as needed. Through active engagement in the Mole Ratio Pogil activity and the use of the corresponding answer key, students can confidently navigate the realm of moles and chemical reactions.
Mole Ratio POGIL Answer Key: Unveiling the Secrets of Chemistry
The Mole Ratio POGIL Answer Key provides students with a comprehensive understanding of the concept of mole ratios in chemistry. Mole ratios play a critical role in stoichiometry, which is the study of the quantitative relationships between reactants and products in a chemical reaction. This answer key unveils the secrets of chemistry by guiding students through various exercises and helping them determine the correct mole ratios for different chemical reactions.
By using the Mole Ratio POGIL Answer Key, students can enhance their problem-solving skills and gain a deeper understanding of how moles, atoms, and molecules interact in chemical reactions. The answer key provides step-by-step explanations and examples that clarify complex concepts, making it easier for students to comprehend the principles behind mole ratios in chemistry.
One of the key benefits of the Mole Ratio POGIL Answer Key is its emphasis on inquiry-based learning. Through guided questions and collaborative activities, students are encouraged to analyze and interpret experimental data, make predictions, and discover patterns. This approach not only helps them develop critical thinking skills but also fosters a deeper appreciation for the scientific method and its applications in chemistry.
- The Mole Ratio POGIL Answer Key also serves as a valuable tool for teachers. With its comprehensive explanations and ready-to-use worksheets, teachers can effectively guide their students through the complex world of mole ratios and stoichiometry. The answer key enables teachers to facilitate engaging classroom discussions, encourage hands-on experiments, and provide individualized support to students.
- The Mole Ratio POGIL Answer Key also serves as a valuable tool for teachers. With its comprehensive explanations and ready-to-use worksheets, teachers can effectively guide their students through the complex world of mole ratios and stoichiometry. The answer key enables teachers to facilitate engaging classroom discussions, encourage hands-on experiments, and provide individualized support to students.
In conclusion, the Mole Ratio POGIL Answer Key is an essential resource for both students and teachers in their exploration of chemistry. By unlocking the secrets of mole ratios, this answer key empowers students to become confident and knowledgeable in the field of chemistry, preparing them for future scientific endeavors.
Understanding Mole Ratios in Chemistry
Mole ratios are a fundamental concept in chemistry that allow us to understand the relationship between the amounts of reactants and products in a chemical reaction. They are based on the idea that chemical reactions occur in specific proportions, which can be calculated using the balanced equation for the reaction.
A mole ratio is a conversion factor that relates the number of moles of one substance to the number of moles of another substance in a reaction. It is determined by the coefficients in the balanced equation. For example, if the balanced equation for a reaction is 2A + 3B -> 4C, the mole ratio between A and B is 2:3, and the mole ratio between A and C is 2:4 or 1:2. This means that for every 2 moles of A, 3 moles of B are used and 4 moles of C are produced.
Understanding mole ratios is important for various calculations in chemistry, such as determining the mass of reactants or products, predicting the yield of a reaction, or determining the limiting reactant. By using mole ratios, scientists can accurately predict the amount of product that will be formed from a given amount of reactants, or vice versa.
When working with mole ratios, it is essential to have a balanced chemical equation. This provides the necessary information about the relative numbers of atoms or molecules involved in the reaction. Without a balanced equation, it would be impossible to determine the correct mole ratios and accurately predict the outcome of a chemical reaction.
In conclusion, mole ratios play a crucial role in understanding and analyzing chemical reactions. They allow scientists to calculate the amounts of reactants and products involved, providing valuable information for various calculations and predictions. Mastery of the concept of mole ratios is essential for success in the field of chemistry.
Importance of Mole Ratio in Chemical Reactions
The mole ratio is an essential concept in chemistry that plays a crucial role in predicting and understanding chemical reactions. It refers to the ratio in which different substances react with each other to form products. The mole ratio is determined by the coefficients in a balanced chemical equation, which represents the stoichiometry of the reaction.
The mole ratio allows chemists to calculate the quantity of reactants and products involved in a chemical reaction. By knowing the mole ratio, they can determine the amount of each substance needed to yield a desired amount of the product or to completely react with another substance. This information is vital in practical applications, such as pharmaceutical synthesis, where precise control of reactant quantities is necessary to optimize the yield and minimize waste.
Furthermore, the mole ratio provides insights into the underlying mechanism of a chemical reaction. It helps chemists understand how atoms rearrange and combine to form new substances by indicating the exact ratio at which they react. This knowledge is crucial in elucidating the pathways and intermediates involved in complex reactions, leading to a better understanding of reaction mechanisms and the design of more efficient and selective reactions.
The mole ratio also empowers scientists to make quantitative predictions about reaction outcomes and properties by relating the amounts of reactants and products. By using the mole ratio, one can calculate the theoretical yield of a product or determine the limiting reagent–the reactant that is completely consumed–and predict the effects of changing reaction conditions, such as temperature or pressure.
In summary, the mole ratio is a fundamental concept in chemistry that allows for accurate measurement, understanding of reaction mechanisms, and predictive power in chemical reactions. It enables chemists to calculate reactant and product quantities, determine stoichiometry, and make predictions about the outcomes and properties of chemical reactions. The mole ratio is an indispensable tool in the field of chemistry, enabling advancements in various areas, from materials science to drug development.
Applying Mole Ratios in Stoichiometry
Stoichiometry is a branch of chemistry that deals with the quantitative relationships between reactants and products in chemical reactions. It involves using mole ratios to calculate the amounts of substances involved in a reaction. Mole ratios are derived from the balanced chemical equation and allow us to convert between different units of measurement, such as moles and grams.
One of the key steps in applying mole ratios is to identify the given and desired quantities. The given quantity is the information provided in the problem, such as the number of moles or grams of a specific substance. The desired quantity is what we want to calculate, which can also be in the form of moles or grams of another substance.
After identifying the given and desired quantities, we can use the mole ratio from the balanced chemical equation to convert between the two. The mole ratio is the ratio of the coefficients in the balanced equation, which represents the number of moles of one substance involved in the reaction relative to another substance.
To apply mole ratios, we use the following steps:
- Write the balanced chemical equation for the reaction.
- Determine the given and desired quantities.
- Use the mole ratio from the balanced equation to calculate the desired quantity.
- Convert the desired quantity to the desired unit of measurement, if necessary.
- Check the units and cancel out any units that are the same on both sides of the equation.
- Calculate the final answer.
By following these steps and using mole ratios, we can accurately calculate the amounts of substances involved in a chemical reaction. This allows us to determine the limiting reactant, predict the amount of product formed, and analyze the efficiency of a reaction. Mole ratios are essential tools in stoichiometry and are widely used in various chemical calculations.
How to Solve Mole Ratio POGIL Worksheets
The Mole Ratio POGIL worksheets are a valuable tool for learning about mole ratios and stoichiometry in chemistry. However, they can sometimes be challenging to solve. Here are some steps to help you solve these worksheets effectively.
1. Understand the problem

Read the problem carefully and make sure you understand what is being asked. Identify the given information and what you are trying to find. Pay attention to any units or coefficients mentioned in the problem.
2. Balance the equation
One of the first steps in solving a mole ratio problem is to balance the chemical equation. Ensure that the number of atoms of each element is the same on both sides of the equation. This step is crucial because mole ratios are based on the coefficients in the balanced equation.
3. Determine the mole ratio
Look at the coefficients in the balanced equation to determine the mole ratio between the reactants and products. The mole ratio indicates the number of moles of one substance involved in the reaction relative to another. It is essential to use the correct mole ratio when solving the problem.
4. Convert units
If necessary, convert the given quantities to moles using the molar mass of the substances involved. This step ensures that all quantities are in the same unit (moles) and allows for easier comparison and calculation.
5. Perform calculations
Use the mole ratio to calculate the unknown quantity. Multiply the given quantity by the appropriate mole ratio to determine the desired result. Make sure to perform any necessary conversions or calculations to obtain the final answer.
By following these steps, you can effectively solve Mole Ratio POGIL worksheets and enhance your understanding of mole ratios and stoichiometry in chemistry.
Analyzing Mole Ratio POGIL Answer Key Samples
When studying the concept of mole ratio in chemistry, it can be helpful to analyze sample answer keys from the POGIL activity. These answer keys provide insights into the correct approach and methodology to solve mole ratio problems.
The mole ratio POGIL answer keys typically consist of a series of questions and corresponding solutions. Each question requires the application of mole ratio principles to determine the desired outcome, such as the number of moles of a reactant or product. By analyzing these answer keys, students can better understand the logical steps involved in solving mole ratio problems.
One common element in the answer keys is the use of balanced chemical equations. These equations provide the necessary information about the mole ratio between the reactants and products. By writing out and correctly balancing the chemical equation, students can identify the appropriate mole ratio to use in their calculations.
Another aspect highlighted in the answer keys is the conversion of units. Mole ratios are often expressed in terms of moles, but the problem might require the determination of mass or volume. Through dimensional analysis, students can convert between different units using the mole ratio as a conversion factor.
Additionally, the answer keys often emphasize the importance of stoichiometry. Stoichiometry deals with the quantitative relationship between reactants and products in a chemical equation. By understanding the stoichiometry of a reaction, students can accurately calculate the mole ratio and determine the desired quantities.
Understanding sample answer keys is a useful tool for mastering mole ratio problems. By analyzing the steps, methods, and concepts used in these answers, students can gain a deeper understanding of mole ratio principles and enhance their problem-solving skills in chemistry.
Common Errors and Misconceptions in Mole Ratio POGIL

When working on the Mole Ratio POGIL activity, students often make some common errors and have misconceptions that can hinder their understanding of the concept. These errors and misconceptions can arise from a lack of familiarity with the topic or from a misunderstanding of the calculations involved.
One common error is the confusion between the mole ratio and the empirical formula. The mole ratio refers to the ratio of moles of one substance to another in a chemical equation, while the empirical formula represents the simplest ratio of atoms in a compound. Students sometimes mistakenly equate these two concepts, leading to incorrect calculations and answers.
Another misconception is the assumption that the mole ratio is always 1:1 for all substances in a chemical equation. While this is true for some reactions, it is not the case for others. Students need to understand that the mole ratio can vary depending on the coefficients in the balanced equation and should be determined using stoichiometry calculations.
Additionally, students often fail to consider the limiting reactant when determining the mole ratio. The limiting reactant is the substance that is completely consumed in a chemical reaction, and its quantity determines the maximum amount of product that can be formed. Ignoring the limiting reactant can lead to incorrect mole ratio calculations and inaccurate predictions of the amount of product produced.
It is important for students to be aware of these common errors and misconceptions when working on the Mole Ratio POGIL activity. By understanding the differences between the mole ratio and empirical formula, considering the stoichiometry calculations, and accounting for the limiting reactant, students can improve their understanding of this fundamental concept in chemistry.