
Understanding the concept of mole-to-grams and grams-to-moles conversions is essential in chemistry. These conversions allow us to convert between the mass of a substance and the number of moles it contains, providing a crucial link between the macroscopic and microscopic worlds of chemistry.
In this worksheet, we will explore different problems that require converting between moles and grams, using the molar mass as a key factor. The molar mass represents the mass of one mole of a substance and is expressed in grams per mole. By knowing the molar mass of a substance, we can convert grams to moles or moles to grams with ease.
The worksheet provides a series of questions that involve calculating the number of moles or grams of a given substance. Students will be given the molar mass or vice versa and will be asked to perform the necessary conversions. Each problem will require the use of the mole-to-grams or grams-to-moles conversion factors to arrive at the correct answer.
By practicing these conversions, students will develop their understanding of the relationship between moles and grams. They will learn to use dimensional analysis to convert between these units and develop the skills required to solve more complex problems in chemistry.
What are moles and grams in chemistry?
In chemistry, moles and grams are units of measurement that are used to quantify the amount of substance present in a sample. These units are essential for calculations and conversions in chemical reactions and other chemical processes.
Moles are a unit of measurement used to express the amount of a substance. One mole is defined as the amount of a substance that contains as many elementary particles (such as atoms, molecules, or ions) as there are atoms in exactly 12 grams of pure carbon-12. The quantity of an element or compound in moles can be determined using its molar mass, which is expressed in grams per mole. The concept of moles is fundamental in chemistry to quantify the number of particles involved in a reaction and to determine the stoichiometry of chemical equations.
Grams are a unit of mass used to measure the amount of a substance. The mass of a substance is often given in grams, and it can be converted to moles using the substance’s molar mass. The molar mass is found by adding up the atomic masses of all the atoms in a molecule. The mass of a substance in grams can be used to calculate the number of moles present. This information is crucial for determining the amount of reactants and products in a chemical reaction.
In summary, moles and grams are essential units of measurement in chemistry for quantifying the amount of substance. Moles are a unit used to express the number of particles in a sample, while grams are used to measure the mass of the substance. These units are interconnected and play a crucial role in understanding and performing calculations in chemistry.
Why are mole to gram conversions important in chemistry?
In chemistry, mole to gram conversions are important because they allow scientists to accurately measure and express the amounts of substances involved in chemical reactions. The mole is a fundamental unit of measurement in chemistry that represents the number of particles (atoms, molecules, ions, etc.) in a given sample. By converting between moles and grams, chemists can determine the mass of a substance and use this information to calculate reaction yields, formulate chemical equations, and determine the stoichiometry of a reaction.
One reason why mole to gram conversions are important is that they allow for precise measurements and comparisons between different substances. Since the mass of an individual atom or molecule is extremely small, it is impractical to work with grams when dealing with large numbers of particles. By converting to moles, chemists can simplify calculations and make it easier to work with quantities on a macroscopic scale. This is particularly helpful when making measurements in the laboratory or conducting experiments.
Furthermore, mole to gram conversions are essential for determining reactant and product quantities in chemical equations. Chemical reactions occur based on specific ratios between reactants and products. By converting between moles and grams, chemists can determine the amount of each substance needed or produced in a reaction. This information is crucial for calculating reaction yields, determining limiting reactants, and understanding the overall balance of a chemical equation.
In summary, mole to gram conversions are important in chemistry because they allow for accurate measurements, simplify calculations, and provide essential information for understanding chemical reactions. By converting between moles and grams, chemists can determine the mass of a substance, calculate reaction yields, and formulate balanced chemical equations. These conversions play a vital role in the field of chemistry and are fundamental to many areas of study and research.
How to Convert Moles to Grams?
Moles and grams are two different units of measurement used in chemistry to quantify the amount of a substance. Moles measure the number of atoms or molecules, while grams measure the mass of a substance. Converting moles to grams involves using the molar mass of the substance, which is the mass of one mole of that substance.
To convert moles to grams, you can follow these steps:
- Identify the substance for which you want to convert moles to grams.
- Determine the molar mass of the substance. This can be found on the periodic table and is usually expressed in grams per mole (g/mol).
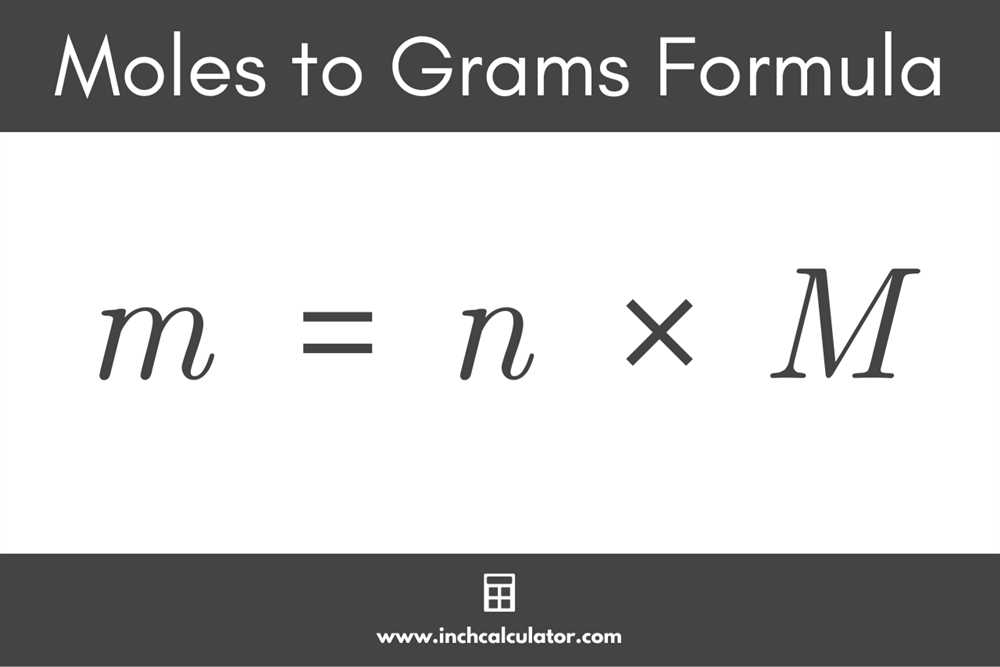
- Take the number of moles you want to convert and multiply it by the molar mass of the substance.
- The result of this calculation will be the mass of the substance in grams.
For example, let’s say you want to convert 2 moles of carbon dioxide (CO2) to grams. The molar mass of carbon dioxide is approximately 44 g/mol. To convert the moles to grams, you would multiply 2 moles by 44 g/mol, resulting in 88 grams of carbon dioxide.
Converting moles to grams is an important skill in chemistry, as it allows you to relate the amount of a substance in moles to its mass in grams. This conversion is often used in chemical calculations and can help determine the amount of a substance needed or produced in a reaction.
Step-by-step guide to convert moles to grams

Converting moles to grams is an essential skill in chemistry. It allows us to relate the amount of substance in moles to its mass in grams. Here is a step-by-step guide to help you convert moles to grams:
- Determine the molar mass: The molar mass is the mass of one mole of a substance. You can find the molar mass by adding up the atomic masses of all the atoms in the chemical formula of the substance.
- Identify the number of moles: Determine the number of moles of the substance you want to convert. This can be given in the problem or can be calculated using the given mass or volume and the molar mass.
- Use the conversion factor: The conversion factor relates the number of moles to the mass in grams. This conversion factor is equal to the molar mass of the substance. Use this conversion factor to convert moles to grams by multiplying the number of moles by the molar mass.
- Calculate the result: Multiply the number of moles by the molar mass to obtain the mass of the substance in grams.
Let’s take an example to illustrate the steps. Suppose we have 2 moles of water (H₂O) and we want to convert it to grams. The molar mass of water is 18.01528 g/mol.
- Step 1: Determine the molar mass of water – which is 18.01528 g/mol.
- Step 2: We already have the number of moles, which is 2 moles.
- Step 3: Use the conversion factor – multiply 2 moles by the molar mass of water (18.01528 g/mol). The moles cancel out, and we are left with 36.03056 grams.
- Step 4: The result is 36.03056 grams.
Following these steps will guide you in converting moles to grams for any substance. Keep in mind the units and the molar mass of the substance, and you’ll be able to successfully convert moles to grams.
Example problems on converting moles to grams
In chemistry, the concept of converting moles to grams is crucial for understanding the relationship between the amount of a substance and its mass. By knowing the molar mass of a compound, we can calculate the mass in grams corresponding to a certain number of moles. Let’s look at some example problems to further illustrate this concept.
Example 1:
Calculate the mass of 2 moles of carbon dioxide (CO2).
Solution:
1. Determine the molar mass of carbon dioxide:
- The molar mass of carbon is 12.01 g/mol
- The molar mass of oxygen is 16.00 g/mol
- Carbon dioxide consists of one carbon atom and two oxygen atoms, so the molar mass is (1 * 12.01 g/mol) + (2 * 16.00 g/mol) = 44.01 g/mol
2. Use the molar mass to calculate the mass of 2 moles of carbon dioxide:
- Mass = number of moles * molar mass
- Mass = 2 moles * 44.01 g/mol
- Mass = 88.02 grams
Example 2:
Find the mass of 0.5 moles of sulfuric acid (H2SO4).
Solution:
1. Determine the molar mass of sulfuric acid:
- The molar mass of hydrogen is 1.01 g/mol
- The molar mass of sulfur is 32.06 g/mol
- The molar mass of oxygen is 16.00 g/mol
- Sulfuric acid consists of 2 hydrogen atoms, 1 sulfur atom, and 4 oxygen atoms, so the molar mass is (2 * 1.01 g/mol) + (1 * 32.06 g/mol) + (4 * 16.00 g/mol) = 98.09 g/mol
2. Use the molar mass to calculate the mass of 0.5 moles of sulfuric acid:
- Mass = number of moles * molar mass
- Mass = 0.5 moles * 98.09 g/mol
- Mass = 49.05 grams
These example problems demonstrate how to convert moles to grams using the molar mass of a compound. By understanding this concept, chemists can accurately determine the mass of substances in various chemical reactions and calculations.
How to convert grams to moles?
Converting grams to moles is an essential calculation in chemistry. It helps determine the amount of substance present in a given sample based on its mass. The conversion involves using the molar mass of the substance, which is the mass of one mole of that substance.
To convert grams to moles, follow these steps:
- Determine the molar mass: Find the atomic masses of all the elements present in the compound and multiply them by the number of atoms in the compound. Add up these masses to get the molar mass.
- Calculate the number of moles: Divide the mass of the sample by the molar mass calculated in the previous step. This will give you the number of moles in the sample.
For example, let’s say we have a sample of water with a mass of 18 grams. The molar mass of water (H2O) is calculated as:
| Element | Atomic Mass (g/mol) | Number of Atoms | Total Mass (g/mol) |
|---|---|---|---|
| Hydrogen (H) | 1.008 | 2 | 2.016 |
| Oxygen (O) | 16.00 | 1 | 16.00 |
| Total molar mass: | 18.016 g/mol | ||
By dividing the mass of the sample (18 g) by the molar mass of water (18.016 g/mol), we find that the sample contains approximately 1 mole of water.
Converting grams to moles is an important concept in stoichiometry and helps chemists in various calculations, such as determining the limiting reactant or calculating the yield of a reaction.
Step-by-step guide to convert grams to moles
In chemistry, it is often necessary to convert between grams and moles, as these are two units commonly used to measure substances. Converting grams to moles allows us to compare different substances based on their molar masses and analyze chemical reactions in terms of the number of moles involved. Here is a step-by-step guide to help you convert grams to moles:
Step 1: Determine the molar mass
The first step in converting grams to moles is to determine the molar mass of the substance you are working with. The molar mass is the mass of one mole of a substance and is expressed in grams per mole. This can be found on the periodic table or calculated by summing the atomic masses of all the elements present in the substance.
Step 2: Convert grams to moles
To convert grams to moles, you need to use the formula:
moles = grams / molar mass
Simply divide the given mass (in grams) by the molar mass (in grams per mole) to obtain the number of moles.
Step 3: Round to the appropriate number of significant figures
After calculating the number of moles, it is important to round the answer to the appropriate number of significant figures, as dictated by the given data or the problem statement.
Step 4: Check your units and calculations
Before finalizing the conversion, it is crucial to double-check your units to ensure they cancel out correctly. Also, go through your calculations to make sure there are no errors or mistakes.
Step 5: Include units in your answer
Always include the appropriate units (moles) in your final answer to clearly indicate the quantity being expressed.
By following these steps, you can accurately convert grams to moles and perform various calculations and analyses in chemistry.
Example problems on converting grams to moles
Converting grams to moles is an essential skill in chemistry. This conversion is based on the molar mass of a substance, which represents the mass of one mole of that substance. To convert grams to moles, you need to divide the given mass of the substance by its molar mass.
Let’s take a look at some example problems to illustrate this concept:
-
Example 1:
Convert 25 grams of water (H2O) to moles.
Solution:
First, we need to determine the molar mass of water. The atomic mass of hydrogen (H) is approximately 1 gram/mole, and the atomic mass of oxygen (O) is approximately 16 grams/mole. Since there are two hydrogen atoms and one oxygen atom in a water molecule, the molar mass of water is 18 grams/mole.
To convert grams to moles, we divide the given mass by the molar mass:
25 grams / 18 grams/mole = 1.39 moles
Therefore, 25 grams of water is equivalent to 1.39 moles.
-
Example 2:
Convert 40 grams of carbon dioxide (CO2) to moles.
Solution:
The molar mass of carbon dioxide can be calculated by adding the atomic masses of carbon (C) and two oxygen (O) atoms. The atomic mass of carbon is approximately 12 grams/mole, and the atomic mass of oxygen is approximately 16 grams/mole. Thus, the molar mass of carbon dioxide is 12 + 2(16) = 44 grams/mole.
Using the formula, we can convert grams to moles:
40 grams / 44 grams/mole = 0.91 moles
Therefore, 40 grams of carbon dioxide is equivalent to 0.91 moles.
These examples demonstrate the process of converting grams to moles using the molar mass of a substance. By knowing the molar mass, you can calculate the number of moles from a given mass and vice versa. This conversion is crucial in various chemistry calculations and helps in understanding the relationships between mass, moles, and particles of a substance.