
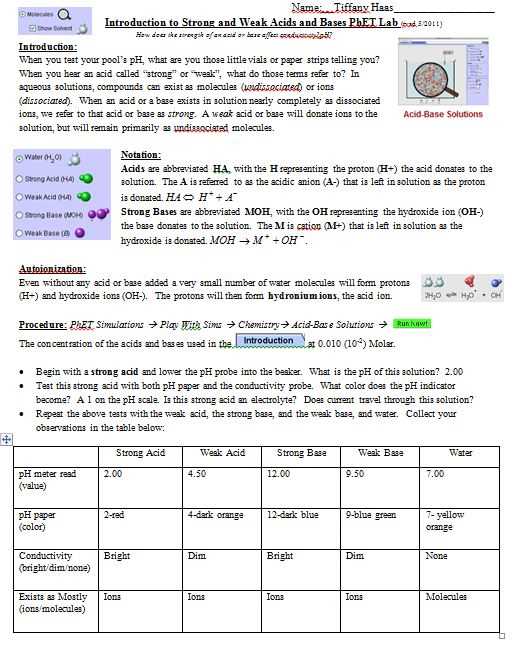
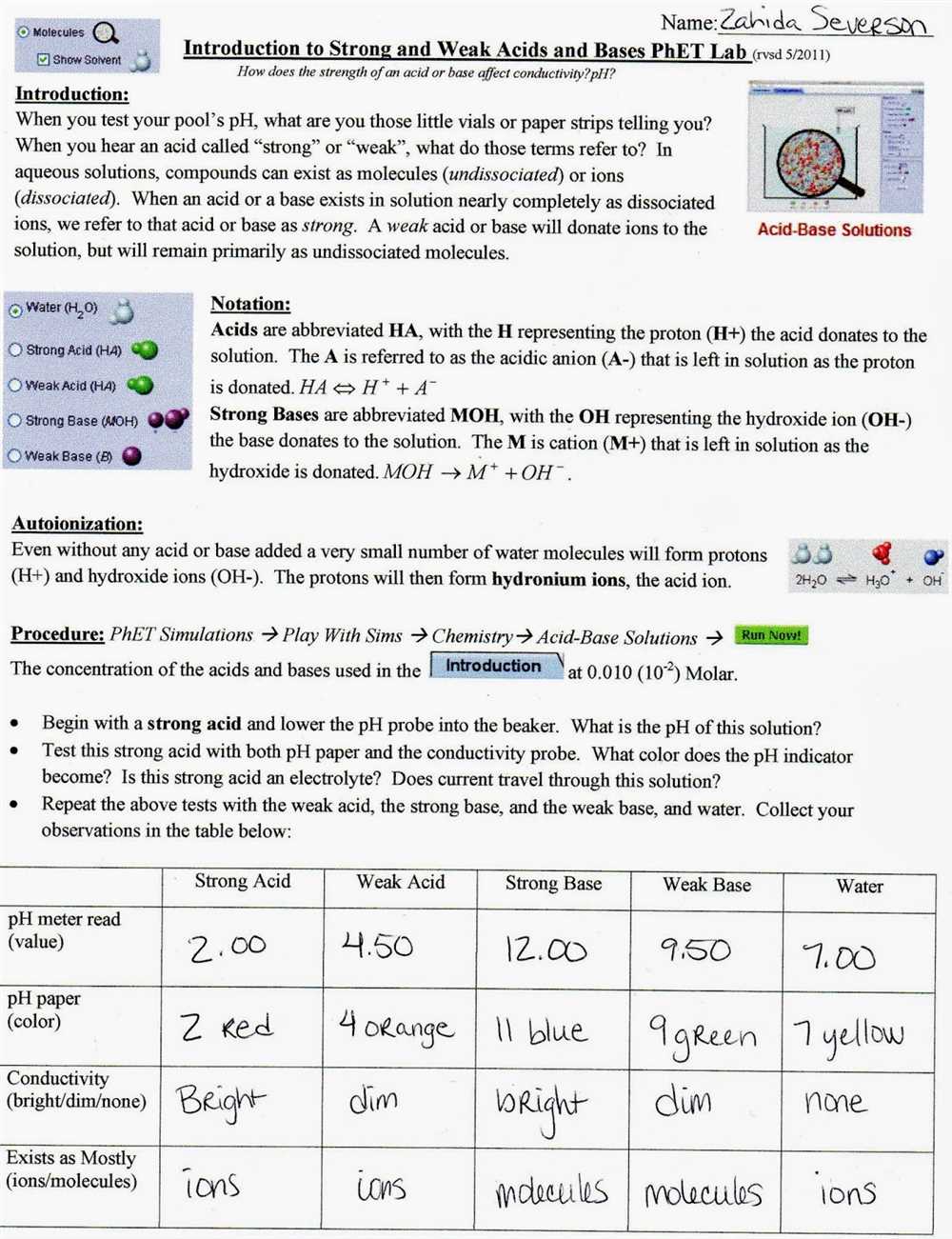
In the study of molecular polarity, the Molecular Polarity PhET Lab provides a hands-on experience for students to explore and understand the concept of molecular polarity. This lab allows students to interact with different molecules, observe their molecular shapes, and determine their polarity through a series of experiments and simulations.
Through this lab, students are able to gain a better understanding of the factors that contribute to molecular polarity, such as the arrangement of atoms and the distribution of charge within a molecule. They also learn how to identify polar and nonpolar molecules based on their molecular shapes and the presence of polar bonds.
The answer key for the Molecular Polarity PhET Lab guides students through the process of determining the polarity of different molecules. It provides step-by-step instructions and explanations for the experiments and simulations conducted in the lab. Additionally, the answer key helps students interpret their observations and analyze the results of their experiments, reinforcing their understanding of molecular polarity.
By using the Molecular Polarity PhET Lab answer key, students can enhance their learning experience, reinforce their knowledge of molecular polarity, and develop their critical thinking and problem-solving skills. This lab serves as a valuable tool in the study of chemistry, allowing students to explore the fascinating world of molecular structures and their interactions.
Molecular Polarity PhET Lab Answer Key
In the Molecular Polarity PhET Lab, students were introduced to the concept of molecular polarity and how it can be determined using a virtual lab simulation on the PhET Interactive Simulations website. The lab allowed students to explore the effect of molecular structure on polarity by manipulating the atoms and bonds in a molecule and observing the resulting molecular geometry and dipole moments.
The answer key for the lab provides students with a guide to understanding the relationship between molecular structure and polarity. It outlines the steps to determine the molecular geometry and the resulting dipole moment for each molecule in the lab. The key also includes a detailed explanation of the reasoning behind each answer, helping students to grasp the underlying concepts.
The answer key serves as a valuable resource for students, allowing them to check their work and verify their understanding of the lab experiment. It provides them with a reference for the correct answers and explanations, enabling them to compare and analyze their own results. In addition, the answer key can be used by teachers as a tool for grading the lab and providing feedback to students.
Key components of the Molecular Polarity PhET Lab Answer Key:
- Step-by-step instructions on determining molecular geometry
- Explanation of the relationship between molecular structure and polarity
- Guidance on calculating dipole moments
- Clarification of the reasoning behind each answer
- Useful resource for students to check their work and understand the concepts
- Grading tool for teachers to assess student understanding
In conclusion, the Molecular Polarity PhET Lab Answer Key provides students with a valuable resource for understanding the relationship between molecular structure and polarity. It offers step-by-step instructions, explanations, and reasoning behind each answer, allowing students to check their work and deepen their understanding of the lab experiment.
What is Molecular Polarity?
Molecular polarity refers to the uneven distribution of electron density within a molecule, resulting in a molecule having a positive and negative end. This imbalance in charge distribution leads to the formation of polar bonds and determines the overall polarity of the molecule.
The polarity of a molecule is influenced by several factors, including the electronegativity difference between atoms, the shape of the molecule, and the presence of lone pairs of electrons.
In a polar covalent bond, atoms with different electronegativities share electrons unequally, resulting in a partial positive charge on the less electronegative atom and a partial negative charge on the more electronegative atom. The polarity of the bonds within a molecule can be represented using arrowhead symbols, with the arrow pointing towards the more electronegative atom.
The overall polarity of a molecule depends on the geometry of the molecule and the arrangement of its polar bonds. If the molecule has a symmetrical shape, such as a linear or tetrahedral shape, the polar bonds may cancel each other out, resulting in a nonpolar molecule. However, if the molecule has an asymmetrical shape, such as a bent or trigonal pyramidal shape, the polar bonds do not cancel each other out, leading to a polar molecule.
The presence of lone pairs of electrons on atoms in a molecule can also contribute to its polarity. Lone pairs tend to occupy more space and create an uneven distribution of electron density, causing the molecule to be polar. Additionally, the presence of multiple polar bonds in a molecule can increase its overall polarity.
Understanding the molecular polarity of a compound is important in various fields, such as chemistry and biology, as it affects the physical and chemical properties of the compound, including its solubility, boiling point, and reactivity. Identifying polar and nonpolar molecules is crucial in predicting the behavior and interactions of compounds in different environments.
How to Determine Molecular Polarity?
Molecular polarity refers to the uneven distribution of electrons within a molecule, which results in a molecule having a positive and negative end. This polarity determines how the molecule interacts with other molecules and its overall behavior. Determining the polarity of a molecule is important in understanding its properties and predicting its chemical reactions. There are several steps involved in determining molecular polarity.
Step 1: Determine the molecular geometry of the molecule. This involves identifying the arrangement of atoms and the spatial orientation of the molecule’s bonds. The molecular geometry can be determined by drawing a Lewis structure or using experimental data.
Step 2: Determine the individual bond polarities of the molecule. Each bond within a molecule has a specific polarity, which is determined by the difference in electronegativity between the atoms involved in the bond. Electronegativity is a measure of an atom’s ability to attract electrons towards itself. The larger the difference in electronegativity between two atoms, the more polar the bond between them.
Step 3: Determine the molecular polarity by considering the overall molecular geometry and the individual bond polarities. If the molecule has a symmetrical shape and all the bond polarities cancel each other out, the molecule is nonpolar. On the other hand, if the molecule has an asymmetrical shape and the bond polarities do not cancel each other out, the molecule is polar.
Overall, determining molecular polarity involves examining the molecular geometry and the bond polarities of a molecule. This information helps in understanding the nature and behavior of molecules, which is crucial in fields such as chemistry and biology.
Step-by-Step Guide to Using the PhET Simulation
PhET simulations are interactive tools developed by the University of Colorado Boulder that allow students to explore various scientific concepts through virtual experiments. One popular simulation is the Molecular Polarity PhET Lab, which helps students understand the polarity of molecules and how it affects their behavior.
To use the Molecular Polarity PhET Lab simulation, follow these step-by-step instructions:
- Access the simulation: Open your web browser and navigate to the PhET website. Search for the “Molecular Polarity” simulation and click on the link to open it.
- Understand the interface: Familiarize yourself with the simulation’s interface. Look for the different tools and options available, such as the molecule builder, the electric field, and the polarity meter.
- Build a molecule: Start by selecting an atom from the periodic table on the left side of the screen. Drag the selected atom to the center of the simulation window to create the molecule. Repeat this step to add more atoms and create a compound.
- Check the bond type: Once you have built a molecule, the simulation will automatically show the type of bonds between the atoms. Use this information to determine if the molecule is polar or nonpolar.
- Measure the dipole moment: Use the polarity meter to measure the dipole moment of the molecule. Move the polarity meter close to different parts of the molecule to see how the dipole moment changes. A higher dipole moment indicates a more polar molecule.
- Apply an electric field: Use the electric field tool to apply an electric field to the molecule. Observe how the molecule aligns itself in response to the field. This will help you understand how polarity affects the behavior of molecules in an electric field.
- Explore different molecules: Repeat the previous steps with different combinations of atoms to explore the polarity of different molecules. Observe the differences in bond types, dipole moments, and response to electric fields.
By following these steps and experimenting with the Molecular Polarity PhET Lab simulation, you will gain a better understanding of molecular polarity and its implications in various chemical and physical processes.
Results and Analysis of the PhET Lab
The PhET Lab on molecular polarity provided valuable insights into the concept of molecular polarity and the factors that influence it. By conducting virtual experiments and manipulating various molecules, we were able to observe the effects of molecular shape, bond polarity, and molecular dipoles on overall molecular polarity.
One of the key observations from the lab was that molecules with symmetrical geometries tend to be nonpolar, while molecules with asymmetrical geometries tend to be polar. This is because in symmetrical molecules, the individual bond dipoles cancel each other out, resulting in a net dipole moment of zero. On the other hand, in asymmetrical molecules, there is an imbalance of electron distribution, leading to a non-zero net dipole moment and thus molecular polarity.
Another important finding was that the presence of polar bonds within a molecule does not always guarantee overall molecular polarity. For example, molecules like carbon dioxide (CO2) and boron trifluoride (BF3) have polar bonds due to differences in electronegativity between the bonded atoms, but their symmetrical geometries result in a net dipole moment of zero and make them nonpolar.
The lab also allowed us to explore the significance of molecular polarity in determining the physical and chemical properties of substances. We learned that polar molecules are attracted to other polar molecules through intermolecular forces such as dipole-dipole interactions, while nonpolar molecules are only influenced by weaker forces like London dispersion forces. This understanding can help explain phenomena such as the dissolving of polar solutes in polar solvents and the higher boiling points of polar substances compared to nonpolar substances.
In conclusion, the PhET Lab on molecular polarity provided a comprehensive and interactive exploration of the factors influencing molecular polarity and its implications. It highlighted the importance of molecular shape and bond polarity in determining overall molecular polarity and helped us understand the role of polarity in intermolecular interactions and substance properties.
Application of Molecular Polarity in Chemistry
Molecular polarity plays a crucial role in various aspects of chemistry, including the study of chemical reactions, solubility, and intermolecular forces. Understanding the polarity of molecules is essential for predicting their behavior and interactions with other substances.
One important application of molecular polarity is in determining the solubility of substances in different solvents. Polar molecules tend to dissolve in polar solvents, while nonpolar molecules dissolve in nonpolar solvents. This concept is fundamental in processes such as extraction and chromatography, where the polarity of different compounds is exploited to separate and purify mixtures.
Chemical reactions
Another key application of molecular polarity is in understanding chemical reactions. Polar molecules are more likely to participate in reactions involving ionic or polar bonds, while nonpolar molecules typically engage in reactions that involve nonpolar covalent bonds. The polarity of molecules affects their reactivity and determines the types of reactions they can undergo. For example, polar solvents can facilitate the dissolution of ionic compounds and promote reactions that involve charged species.
Intermolecular forces
The polarity of molecules also influences the strength of intermolecular forces. In general, polar molecules have stronger intermolecular forces compared to nonpolar molecules. These forces include dipole-dipole interactions and hydrogen bonding. The strength of intermolecular forces affects properties such as boiling points, melting points, and viscosity of substances.
Predicting molecular properties
Additionally, understanding molecular polarity allows scientists to predict and explain various molecular properties. For instance, the presence of polar bonds in a molecule leads to a non-zero dipole moment, which can be used to predict the overall polarity of the molecule. This information is valuable in fields such as material science, drug design, and environmental chemistry.
Conclusion
In conclusion, the concept of molecular polarity has wide-ranging applications in chemistry. It is crucial for determining the solubility of substances, understanding chemical reactions, predicting intermolecular forces, and explaining molecular properties. By analyzing the polarity of molecules, scientists can better comprehend and manipulate chemical processes and create new materials with specific characteristics.
The Importance of Understanding Molecular Polarity
Understanding molecular polarity is crucial in various scientific disciplines, especially in the fields of chemistry and biology. Molecular polarity refers to the distribution of electrical charge within a molecule, which significantly impacts its physical and chemical properties.
Chemical Reactions: The knowledge of molecular polarity helps chemists predict how different molecules will interact and undergo chemical reactions. Polar molecules tend to interact with other polar and charged molecules, while nonpolar molecules preferentially interact with other nonpolar molecules. By understanding the polarity of molecules involved in a reaction, scientists can optimize reaction conditions and predict the outcome of chemical reactions.
Biological Systems: In biology, molecular polarity plays a crucial role in various biological processes. For example, the polarity of water molecules allows them to form hydrogen bonds with other polar molecules, facilitating the transport of nutrients and waste products across cell membranes. Additionally, understanding the polarity of biomolecules, such as proteins and DNA, is essential for studying their structure and function.
Physical Properties: Molecular polarity also affects the physical properties of substances. For instance, polar molecules tend to have higher boiling and melting points compared to nonpolar molecules due to the presence of intermolecular forces, such as dipole-dipole interactions and hydrogen bonding. Additionally, the solubility of a substance in different solvents is influenced by the polarity of both the solute and the solvent.
Drug Design: Molecular polarity is crucial in drug design and development. By understanding the polarity of a drug molecule, scientists can predict its solubility, absorption, distribution, metabolism, and excretion within the body. This knowledge helps in designing drugs with optimal properties, such as improved bioavailability and target specificity.
In conclusion, understanding molecular polarity is of utmost importance in various scientific domains. It enables scientists to predict chemical reactions, study biological processes, determine physical properties of substances, and design drugs with enhanced therapeutic potential. By unraveling the complex nature of molecular polarity, scientists continue to advance our knowledge and make groundbreaking discoveries in numerous fields.