
Understanding the polarity of molecules is crucial in the field of chemistry, as it helps determine their properties and behaviors. The Molecule Polarity Phet simulation is a powerful tool that allows students to explore the concept of molecule polarity and understand how different factors influence the overall polarity of a molecule.
In this lab activity, students are introduced to the concept of polarity and its impact on a molecule’s physical and chemical properties. By using the Molecule Polarity Phet simulation, students can manipulate different atoms and bonds to create various molecular structures and observe their respective polarities.
The answer key to the Molecule Polarity Phet simulation provides students with a comprehensive guide to understanding and analyzing the polarity of different molecules. It offers explanations for the polarity of each molecule, highlighting the bond dipoles and molecular geometry that contribute to the overall polarity.
By using the answer key, students can identify the relationship between the electronegativity of atoms, the shape of the molecule, and its polarity. They can also learn how to determine the polarity of a molecule based on the presence of lone pairs of electrons and asymmetrical molecular structures.
Overall, the Molecule Polarity Phet Answer Key serves as a valuable resource for students to reinforce their understanding of molecule polarity and its significance in the world of chemistry. With a clear and concise explanation of molecular structures and their polarities, students can confidently apply their knowledge to predict the behavior and properties of various compounds.
Molecule Polarity PhET Answer Key
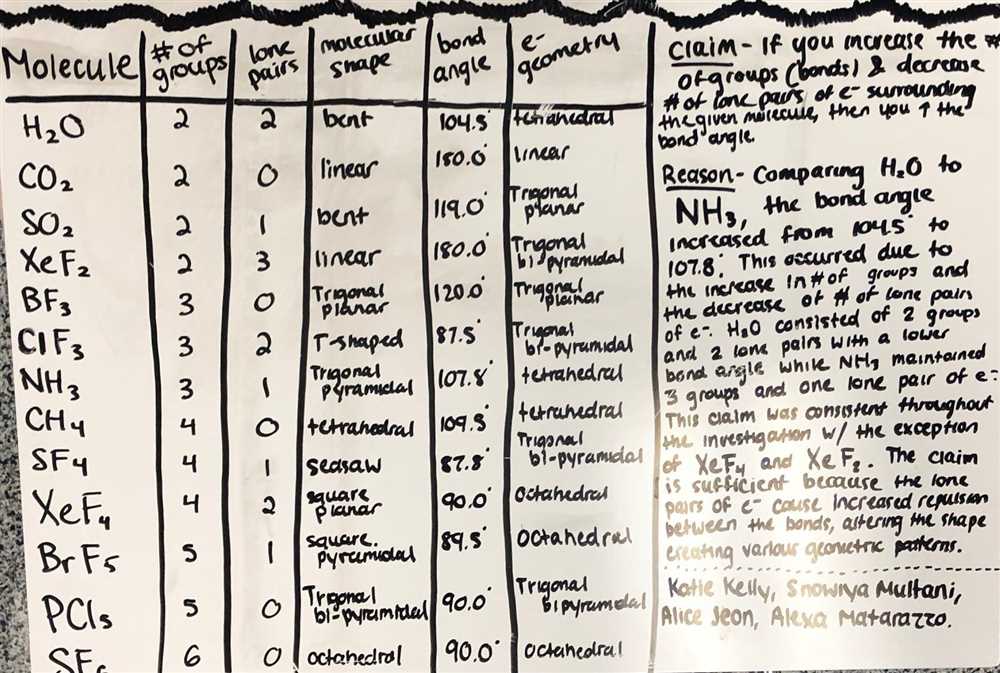
The Molecule Polarity PhET Answer Key is a useful resource for understanding the concept of molecular polarity. This answer key provides explanations and answers to the questions within the PhET simulation on molecule polarity.
The PhET simulation allows users to explore how different molecules interact with each other and how the arrangement of atoms within a molecule can affect its polarity. In this simulation, users can manipulate the atoms and bonds of various molecules and observe how these changes affect the overall polarity of the molecule.
The Molecule Polarity PhET Answer Key provides step-by-step explanations to help users understand the underlying principles behind molecular polarity. It covers topics such as determining the polarity of a molecule based on its geometry, identifying polar and nonpolar bonds, and understanding the relationship between electronegativity and molecular polarity.
The answer key also includes detailed diagrams and examples to illustrate the concepts being discussed. This visual representation helps users visualize the structural characteristics of different molecules and how they contribute to their overall polarity.
The Molecule Polarity PhET Answer Key is a valuable tool for students and educators studying chemistry or related fields. It provides a comprehensive understanding of molecular polarity and helps users develop their problem-solving skills in this area.
Understanding Molecule Polarity
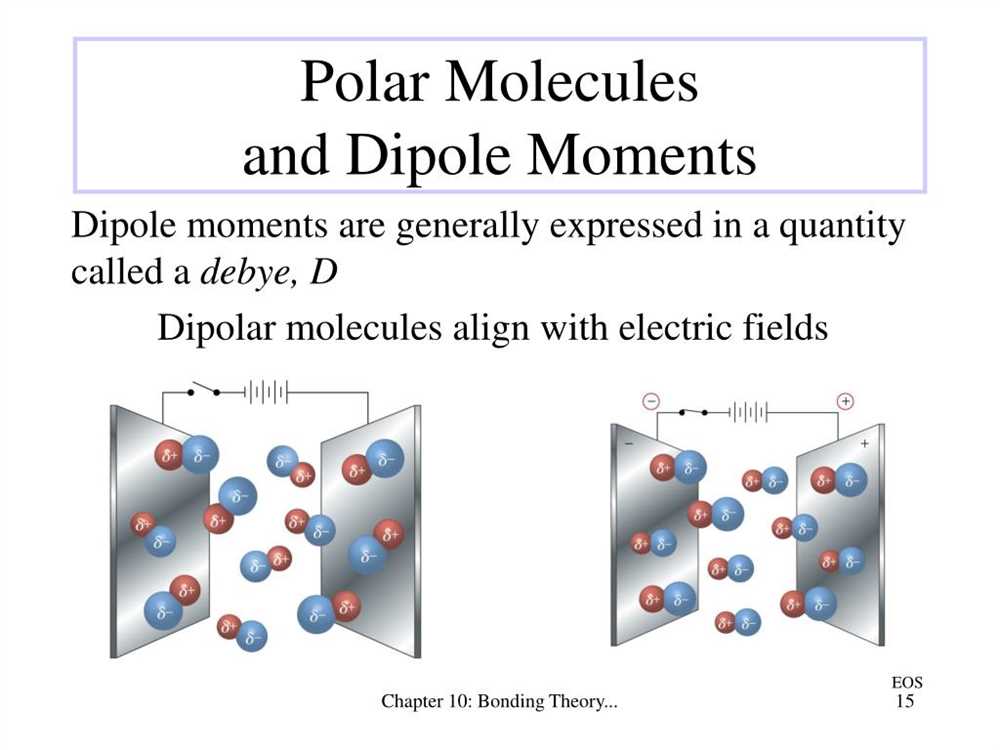
Molecule polarity is a concept in chemistry that refers to the distribution of charge within a molecule. It is important to understand because it affects various properties of molecules, such as solubility, boiling points, and interactions with other molecules. Molecule polarity is determined by the presence of polar bonds and the overall shape of the molecule.
A polar bond is a type of chemical bond where the electrons are not shared equally between the atoms. One atom tends to attract the electrons more strongly, creating a partial positive charge on one side of the bond and a partial negative charge on the other side. This creates a dipole moment in the bond, making it polar. The presence of polar bonds in a molecule does not guarantee that the molecule is polar, as the overall shape of the molecule also plays a significant role.
In determining the overall polarity of a molecule, we consider both the individual bond polarities and the molecular geometry. If a molecule has polar bonds and is symmetrically arranged, the bond polarities can cancel each other out, resulting in a nonpolar molecule. On the other hand, if a molecule has polar bonds and an asymmetrical molecular geometry, the bond polarities do not cancel each other out, leading to a polar molecule.
Understanding molecule polarity is important in many areas of chemistry, such as organic chemistry, biochemistry, and materials science. It allows us to predict the behavior of molecules, their interactions with other molecules, and their suitability for specific applications. Moreover, it provides insights into the structure-function relationships of compounds and helps in the design of new materials with desired properties.
What is PhET?

PhET is a name that stands for Physics Education Technology. It is a project started at the University of Colorado Boulder, with the aim of creating interactive simulations for teaching and learning science. These simulations, or “PhET sims,” are designed to help students understand and visualize complex scientific concepts through hands-on exploration and experimentation. They cover a wide range of topics in physics, chemistry, biology, earth science, mathematics, and engineering.
PhET sims are freely available for anyone to use and are widely used in classrooms around the world. They are ideal for both in-class demonstrations and individual student exploration. The simulations provide a virtual environment where students can manipulate variables, observe outcomes, and test hypotheses. This interactive approach helps students build a deeper understanding of scientific principles and develop critical thinking and problem-solving skills.
PhET sims are designed to be intuitive and user-friendly, making them accessible to students of all ages and abilities. They are available in multiple languages and can be easily accessed online through the PhET website or downloaded for offline use. Teachers can also incorporate PhET sims into their lesson plans, using the provided teacher resources and lesson ideas to enhance their instruction.
PhET has received numerous awards and recognition for its contributions to science education. The project continues to evolve and expand, with new simulations being added regularly and ongoing research to improve the effectiveness of the sims in teaching and learning. Whether used in a classroom setting or for self-study, PhET sims provide a valuable tool for engaging students and promoting a deeper understanding of scientific concepts.
Using PhET to Explore Molecule Polarity
PhET simulations offer a valuable tool for exploring and understanding complex scientific concepts, such as molecule polarity. Molecule polarity refers to the uneven distribution of charge in a molecule, leading to the presence of a positive and negative region. Understanding the polarity of molecules is crucial in various fields, including chemistry, biology, and environmental science.
One of the PhET simulations that can aid in exploring molecule polarity is the “Molecule Polarity” simulation. This interactive simulation allows users to build and manipulate molecules using different atoms and examine their resulting polarity. Through the simulation, users can learn about various factors that contribute to molecular polarity, including electronegativity, molecular shape, and bond polarity.
Electronegativity plays a significant role in determining the polarity of a molecule. Atoms with higher electronegativity attract electrons more strongly, creating a partial negative charge, while atoms with lower electronegativity have a partial positive charge. By manipulating the electronegativity of different atoms in the simulation, users can observe how it affects the overall polarity of the molecule.
Molecular shape also influences molecule polarity. Symmetrical molecules, such as linear or nonpolar ones, have an equal distribution of charge and are nonpolar. However, asymmetrical molecules, such as bent or polar ones, have an uneven distribution of charge, resulting in polarity. The simulation allows users to experiment with different molecular shapes, providing a visual representation of how shape impacts polarity.
- The simulation also illustrates the concept of bond polarity. Covalent bonds can be polar or nonpolar, depending on the electronegativity difference between the atoms involved. By manipulating the type of bond (nonpolar or polar) in the simulation, users can observe how it affects the overall polarity of the molecule.
- The “Molecule Polarity” simulation provides an interactive and engaging way to explore and understand the concept of molecule polarity. It allows users to manipulate various factors, such as electronegativity, molecular shape, and bond polarity, to observe their impact on the overall polarity of a molecule. By using PhET simulations, learners can develop a deeper comprehension of this fundamental concept in chemistry and related fields.
Importance of Understanding Molecule Polarity
Molecule polarity is a fundamental concept in chemistry that plays a crucial role in understanding various chemical processes and phenomena. It refers to the distribution of charges within a molecule, which determines its overall polarity. The understanding of molecule polarity is important for several reasons.
Firstly, molecule polarity affects the physical and chemical properties of substances. Polar molecules have an uneven distribution of charge, with certain regions being more positively or negatively charged. This uneven distribution gives rise to unique properties, such as the ability to form hydrogen bonds or dissolve in polar solvents. Nonpolar molecules, on the other hand, have an even distribution of charge and exhibit different properties. Understanding the polarity of molecules helps in predicting and explaining these properties, which is crucial in various fields, including medicine, materials science, and environmental science.
Furthermore, an understanding of molecule polarity is essential for understanding chemical reactions. The polarity of the reactants and products influences the nature and strength of the intermolecular forces involved, which in turn affects the reaction kinetics and thermodynamics. For example, polar molecules tend to undergo reactions involving intermolecular forces, such as hydrogen bonding or dipole-dipole interactions. Nonpolar molecules, on the other hand, may undergo reactions involving weaker forces, such as London dispersion forces. By knowing the polarity of the molecules involved, chemists can better predict and control the outcome of chemical reactions.
In addition, understanding molecule polarity is crucial for the study of molecular geometry and the three-dimensional arrangement of atoms in a molecule. The polarity of individual bonds and the overall molecular structure determine the molecular shape and symmetry, which in turn affects the molecule’s physical and chemical properties. For instance, molecules with polar bonds but a symmetrical shape can be nonpolar overall, while molecules with polar bonds and an asymmetrical shape are generally polar. The knowledge of molecule polarity helps in determining the molecular geometry and understanding how it relates to a molecule’s properties and behavior.
In conclusion, understanding molecule polarity is of utmost importance in chemistry as it provides insights into the physical and chemical properties of substances, influences chemical reactions, and helps in determining molecular geometry. By understanding molecule polarity, scientists and researchers can make informed decisions, design new materials, and develop efficient chemical processes.
Molecule Polarity PhET Activity
In the Molecule Polarity PhET activity, students explore the concept of molecular polarity and its relationship to molecular structure. The activity begins by introducing the concept of electronegativity and how it affects the distribution of electrons in a molecule. Through interactive simulations, students can manipulate the shape and polarity of different molecules by changing their composition and bond angles.
One key aspect of the activity is the exploration of different types of molecular geometries, such as linear, bent, and trigonal pyramidal. By manipulating these geometries, students can observe how the distribution of polar bonds can result in a polar or nonpolar molecule. They can also investigate the effects of different electron pair arrangements on the overall polarity of a molecule.
The activity provides students with a hands-on experience in understanding molecular polarity and its relationship to the physical properties of substances. By manipulating the simulations and observing their effects on the molecules, students can develop a deeper understanding of the concept and its implications in various fields such as chemistry, biology, and materials science.
- Explore the concept of molecule polarity
- Manipulate molecular structure and observe the effects on polarity
- Investigate different types of molecular geometries
- Understand the relationship between electronegativity and molecular polarity
- Gain a practical understanding of molecular polarity through interactive simulations
Common Misconceptions about Molecule Polarity
Understanding molecule polarity is crucial in various scientific fields, such as chemistry and biology. However, there are several common misconceptions that can lead to misunderstandings about molecule polarity and its implications. It is important to address these misconceptions to build a strong foundation in understanding this concept.
1. Molecules with polar bonds are always polar. One common misconception is that if a molecule contains polar bonds, it is always polar overall. While polar bonds contribute to the overall polarity of a molecule, other factors, such as the molecule’s geometry and the distribution of its charges, also play a significant role. It is essential to consider the molecular shape and the presence of any lone pairs of electrons to determine the molecule’s polarity accurately.
2. Nonpolar molecules cannot contain polar bonds. Another misconception is that nonpolar molecules cannot have polar bonds. In reality, a molecule can have polar bonds if the electronegativity difference between the atoms forming the bond is significant enough. However, if the polar bonds are symmetrically arranged in a molecule’s structure, the bond polarities may cancel out, resulting in a nonpolar molecule. An example of this is carbon dioxide (CO2), which has polar C-O bonds but is a nonpolar molecule due to its linear shape.
It is essential to debunk these misconceptions to obtain a clear understanding of molecule polarity. By considering various factors such as bond type, molecular shape, and charge distribution, one can accurately determine the polarity of a molecule and predict its chemical behavior. A strong foundation in molecule polarity is crucial for further studies and applications in the field of science.